Session Information
Date: Monday, October 27, 2025
Title: (1612–1632) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Given the increasing number of patients with giant cell arteritis (GCA) in Japan and the higher incidence of Takayasu arteritis (TAK) compared to Europe and the U.S., our aim is to validate the discriminability of the modified 2022 ACR/EULAR GCA classification criteria for TAK and GCA and to compare the clinical profiles of GCA and TAK reclassified by the classification criteria for large-vessel vasculitis (LVV).
Methods: The Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis (JPVAS) prospective study included 117 incidental GCA and 74 TAK diagnosed by attending physicians at participating JPVAS facilities from 2015 to 2019. In the modified 2022 ACR/EULAR GCA classification criteria, shoulder or neck stiffness was changed to polymyalgia rheumatica (PMR), and fluorodeoxyglucose (FDG)-positron emission tomography (PET) activity to wall thickening, stenosis, or aneurysm of the descending thoracic to abdominal aorta. We applied the GCA criteria first to our cohort, followed by the JPVAS diagnostic criteria for TAK, in which patients are classified as TAK if they exhibit at least one clinical symptom along with diffuse or multiple arterial lesions including wall thickening.
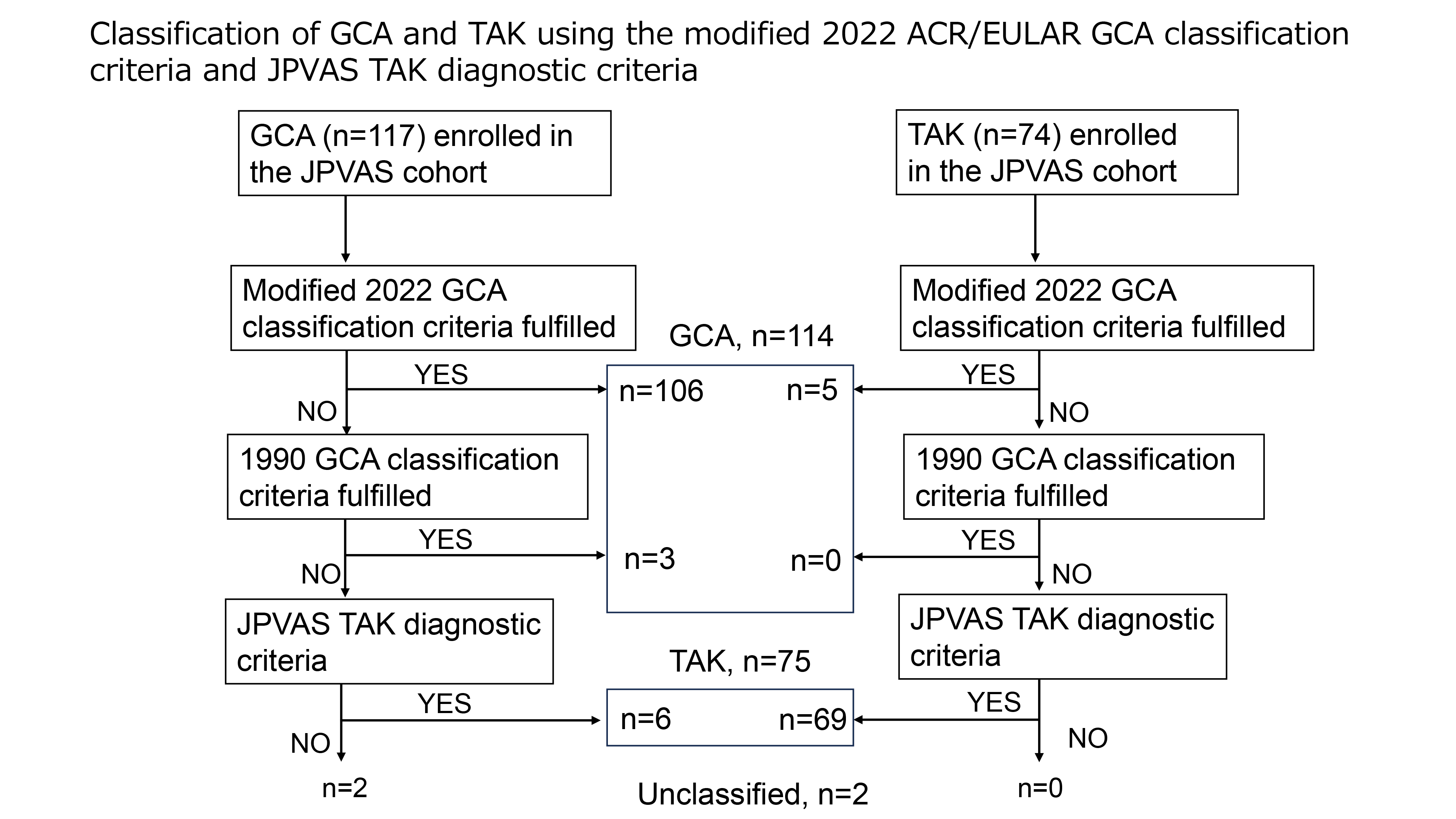
Results: The modified GCA classification criteria had sensitivities of 91.5% for overall GCA, 90.9% for large-vessel GCA (LV-GCA), and 36.3% for isolated LV-GCA without cranial lesions. The specificity was 93.2%. Discrimination between GCA and TAK using the modified criteria excluding age was high, with a C statistic of 0.945 (95% CI: 0.916–0.975). Combining PMR, bilateral axillary arteries, descending thoracic to abdominal aortic lesions, and inflammatory markers (i.e., using only these four items out of original 11 items) yielded a C statistic of 0.730 (95% CI: 0.661–0.799). Excluding PMR from this combination resulted in poor discrimination of 0.598 (95% CI: 0.530–0.667); adding age 50 years or older improved the discrimination to 0.898 (95% CI: 0.847–0.949). Out of 117 GCA and 74 TAK patients enrolled in the JPVAS, 106 and five were classified as GCA based on the modified 2022 GCA classification criteria, followed by the 1990 ACR classification criteria and the JPVAS criteria, and overall, 114, 75, and two were classified as GCA, TAK, and unclassified, respectively (Figure 1). A comparison of GCA and TAK after the classification revealed notable differences in age and clinical features (Table 1). Bilateral axillary artery and descending thoracic to abdominal aorta were more common lesions in LV-GCA than in TAK. Arterial stenosis was significantly more prevalent in TAK than in LV-GCA (Table 2). Among the five patients with TAK classified as GCA, two were under 60, one had carotid artery tenderness, three had bilateral axillary artery lesions, and two were HLA-B52 positive.
Conclusion: The discriminability of the modified 2022 ACR/EULAR GCA classification criteria for TAK and GCA was good, and the classification utilizing the modified GCA criteria and the JPVAS TAK criteria was seen as beneficial. However, the 1990 ACR classification criteria was needed for a few cases, and classification of late-onset TAK and LV-GCA was difficult in some cases.
To cite this abstract in AMA style:
Sugihara T, Harigai M, Uchida H, Maejima Y, Yoshifuji H, Watanabe Y, Onishi Y, Furuta S, Ishii T, Shirai T, Naniwa T, Ogawa N, Kaneko Y, Dobashi H, Amano K, Ishizaki J, Miyamae T, Ito S, Komagata Y, Tamura N, Nakaoka Y. Validation of the classification algorithm using the modified 2022 ACR/EULAR giant cell arteritis classification criteria and Japanese Takayasu arteritis diagnostic criteria [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/validation-of-the-classification-algorithm-using-the-modified-2022-acr-eular-giant-cell-arteritis-classification-criteria-and-japanese-takayasu-arteritis-diagnostic-criteria/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-the-classification-algorithm-using-the-modified-2022-acr-eular-giant-cell-arteritis-classification-criteria-and-japanese-takayasu-arteritis-diagnostic-criteria/

.jpg)
.jpg)