Session Information
Date: Monday, October 27, 2025
Title: (1612–1632) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: A previous post hoc analysis of frail (defined by claims-based frailty index [CFI] ≥0.2) patients with PMR on second line (2L) and 3L treatment showed that those on IL-6 receptor inhibitors (IL-6Ri) were more than twice (HR 2.32) as likely to discontinue GC vs. those on conventional synthetic immunomodulators (csIM: methotrexate [MTX], azathioprine, and leflunomide), nearly 2 times the effect size in overall PMR cohort (HR 1.28). This study builds on prior analysis by comparing IL-6Ri to MTX as 2L treatment, with frailty as inclusion criteria and level of frailty as PS match covariate. Frailty was defined as CFI ≥0.25 (validated threshold in the general population), minimizing inclusion of patients with pre-frailty. This study compared effectiveness of IL-6Ri to MTX in GC-refractory frail patients with PMR.
Methods: This observational retrospective comparative cohort study compared effectiveness of IL-6Ri to MTX using US Medicare claims data (10/2016-12/2022). Inclusion criteria were age ≥50 years, ≥1 inpatient or ≥2 outpatient PMR diagnosis codes ≥30 days apart, ≤25 mg prednisone equivalent dose (PED), CFI ≥0.25, initiation of IL-6Ri or MTX (index date) with no prior IL-6Ri/csIM and continuous enrollment 180 days prior (baseline) to 1 day. Patients initiating IL-6Ri and csIM on same day and patients with seropositive RA, other systemic rheumatic disease (except giant cell arteritis), organ transplant, multiple sclerosis or active malignancy treatment during baseline were excluded. MTX initiators were direct matched on select characteristics and then PS matched on demographic, treatment and clinical characteristics. Primary and key secondary outcomes were time to GC discontinuation ( >60-day gap) and time to minimal (≤2 mg/day PED) or no GC, respectively.
Results: Overall, 29% and 18.3% of patients in IL-6Ri and MTX groups, respectively, had CFI ≥0.25. Of these, 86 matched pairs receiving 2L treatment with no prior IL-6Ri were identified. Patient characteristics were generally balanced, though the mean time from first PMR diagnosis to index date (1025 vs. 799 days) and occurrence of seronegative RA (32.6% vs. 22.1%) were higher in IL-6Ri vs. MTX groups. About 1/4th of the patients were moderately-severely frail (CFI ≥0.35, 20.9% vs. 26.7%) (Table 1). Time to GC discontinuation (Figure 1A) and time to minimal or no GC (Figure 1B) up to 1 year were significantly shorter for IL-6Ri vs. MTX-treated patients (HR [95% CI] 1.75 [1.05, 2.93]; p=0.033) and (HR [95% CI] 1.86 [1.15, 3.00]; p=0.011), respectively.
Conclusion: IL-6Ri therapy led to a more rapid GC discontinuation or to achieving minimal GC dose in frail patients with PMR, suggesting that this vulnerable population may benefit more from IL-6Ri vs. MTX.Original abstract at EULAR 2025.
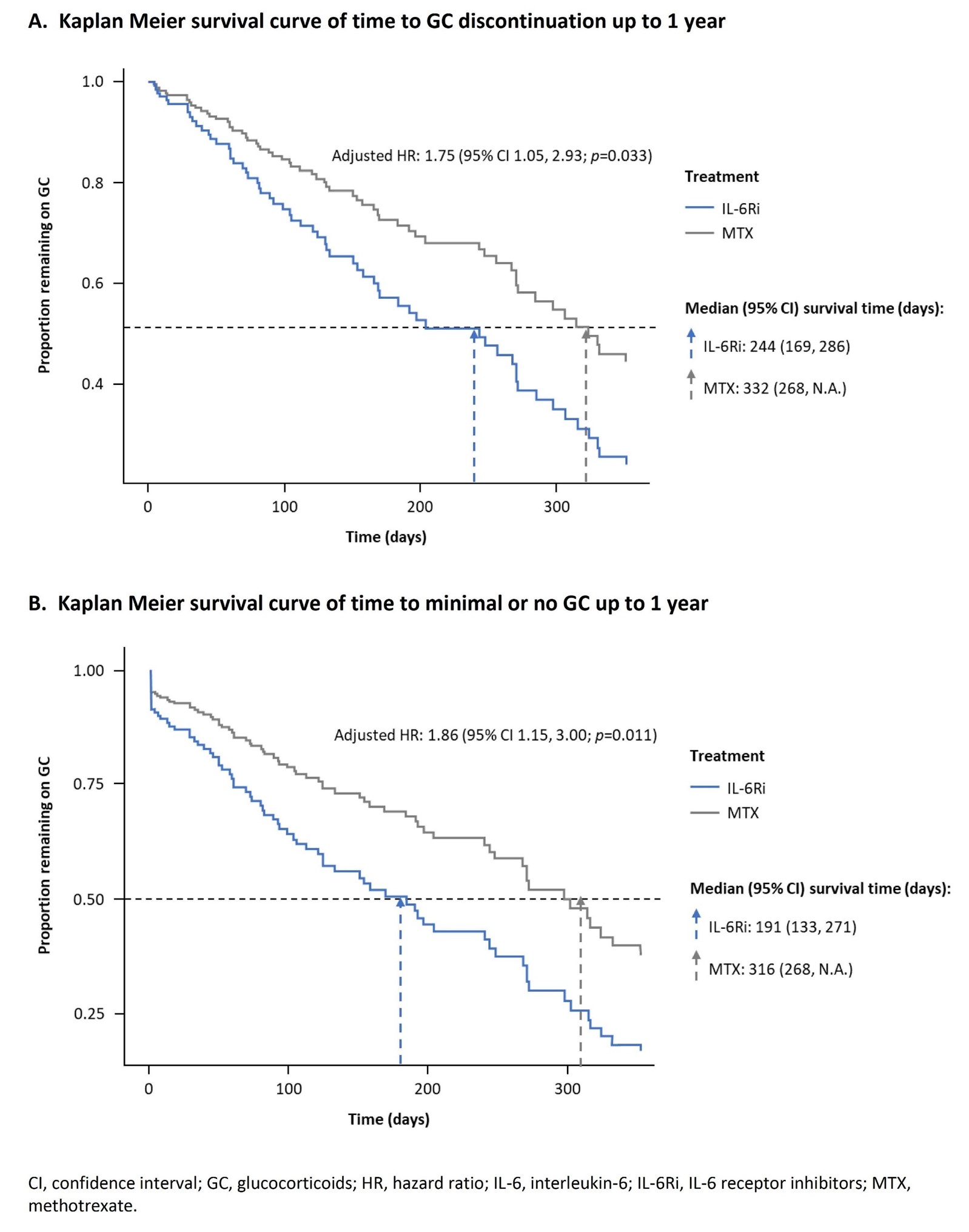 Table 1. Post propensity score match patient characteristics.
Table 1. Post propensity score match patient characteristics.
To cite this abstract in AMA style:
Sattui S, Dejaco C, Ford K, Fiore S, Unizony S, Xie F, Curtis J. Real-World Effectiveness of Interleukin-6 Receptor Inhibitors Compared to Methotrexate in Steroid-Refractory Frail Patients with Polymyalgia Rheumatica [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-effectiveness-of-interleukin-6-receptor-inhibitors-compared-to-methotrexate-in-steroid-refractory-frail-patients-with-polymyalgia-rheumatica/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-effectiveness-of-interleukin-6-receptor-inhibitors-compared-to-methotrexate-in-steroid-refractory-frail-patients-with-polymyalgia-rheumatica/

.jpg)