Session Information
Date: Monday, October 27, 2025
Title: (1612–1632) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: EULAR recommendations informing the management of Polymyalgia Rheumatica (PMR) and Large Vessel Vasculitis (LVV) were previously published separately in 2015 [1] and 2020 [2] respectively. Newly-emerging evidence has necessitated a comprehensive update. We aimed to conduct a systematic literature review (SLR) to inform the 2025 EULAR recommendations for PMR and LVV.
Methods: An SLR (PROSPERO registration: CRD42024560403), performed on 31st December 2024 on MEDLINE, EMBASE and CENTRAL searched for literature on adult patients with PMR, Giant Cell Arteritis (GCA) or Takayasu arteritis (TAK), using MeSH terms for pharmacological and non-pharmacological management. Eligible studies included randomized controlled trials (RCTs) and observational studies, published since 1st April 2014 (for PMR) or 31st December 2017 (for LVV), without language limitations. Abstracts presented at EULAR 2024 and ACR 2024 were additionally hand-searched. Studies were assessed for their quality using relevant Risk of Bias (RoB) tools for RCTs/observational studies (Cochrane Risk of Bias 2/Newcastle Ottawa Scale).
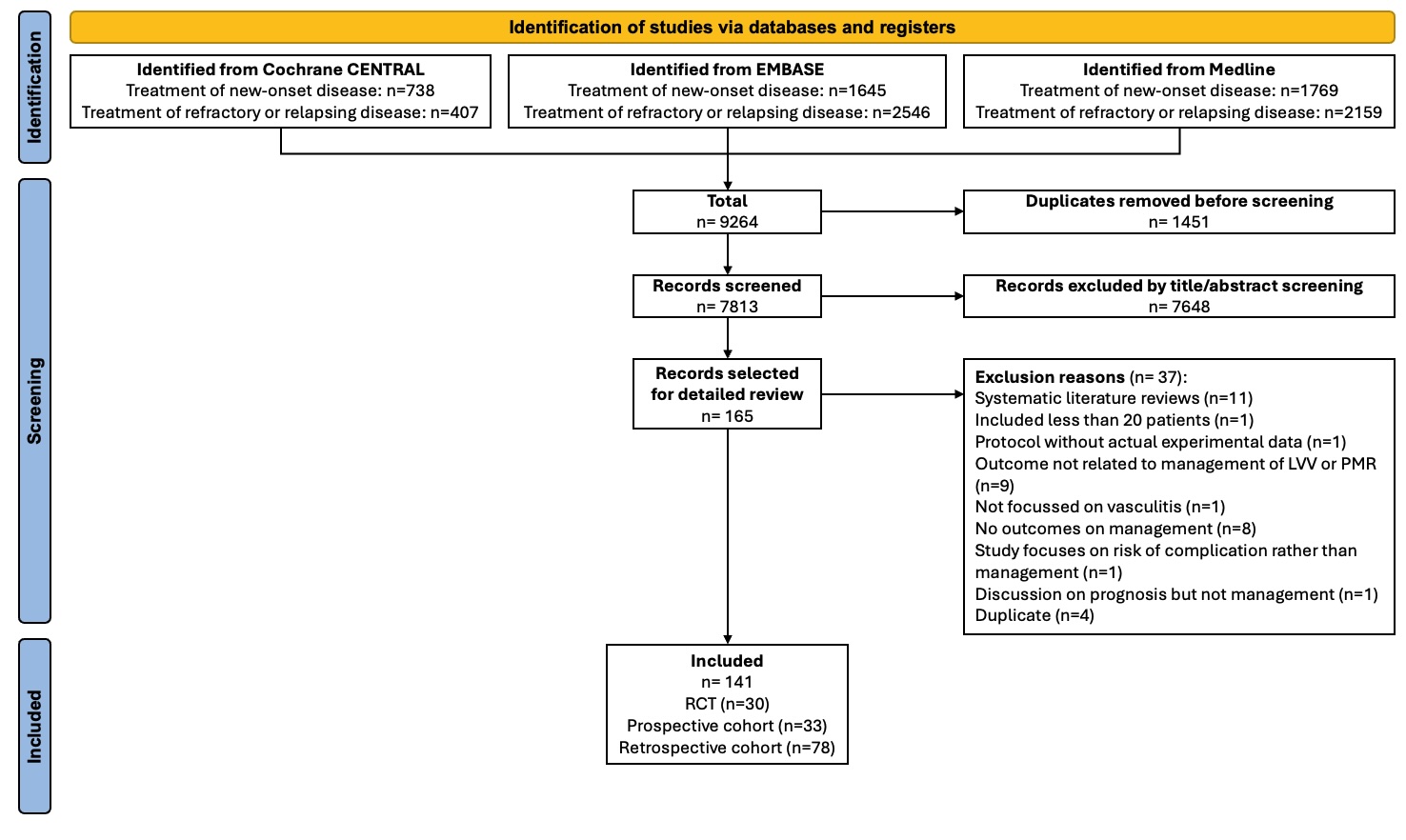
Results: After deduplication and full-text screening, 141 articles were included (30 RCTs) (Figure 1). RCTs of GCA (low RoB) reported superiority of secukinumab (Phase-2, n=1), mavrilimumab (Phase-2, n=1) and upadacitinib (Phase-3, n=1) vs placebo; Phase-2 trials of sarilumab (n=1) and sirukumab (n=1) were prematurely terminated (high RoB). The two RCTs of sariliumab and sirukumab in GCA were at high risk of bias due to their premature termination due to which no inferences could be drawn about their effectiveness. Secondary analyses from the GiACTA trial reported similar efficacy of tocilizumab versus placebo in those with cranial symptoms, PMR symptoms, or both cranial & PMR symptoms, and better improvements in SF-36 and fatigue scores with tocilizumab than placebo. RCTs of TAK reported similar effectiveness of methotrexate or mycophenolate (unclear RoB), and superiority of adalimumab vs tocilizumab (high RoB). Phase-3 RCTs in PMR revealed superiority of tocilizumab and sarilumab (low RoB) vs. placebo while smaller phase-2 studies provided initial evidence for efficacy of rituximab (low RoB) and abatacept (unclear RoB) vs placebo (Table 1). A RCT showed similar efficacy of tofacitinib or glucocorticoids in PMR although this study was at high risk of bias.
Conclusion: The SLR identified several new Phase-2 and Phase-3 RCTs about the efficacy and safety of IL-6i, IL-17i, Janus kinase inhibitors and B-cell depletion therapies in GCA and PMR. High quality adequately sized RCTs in TAK are still lacking. This evidence was used to inform the 2025 EULAR recommendations for management of Polymyalgia Rheumatica and Large Vessel Vasculitis.References1. Dejaco C, et al. Ann Rheum Dis. 2015 Oct;74(10):1799-807.2. Hellmich B, et al. Ann Rheum Dis. 2020 Jan;79(1):19-30.
.jpg) Table 1: Low Risk of Bias RCTs of patients with GCA or PMR
Table 1: Low Risk of Bias RCTs of patients with GCA or PMR
To cite this abstract in AMA style:
Dey M, Misra D, Bartoletti A, Ducker G, Monti S, Hellmich B, Mukhtyar C, Studenic P, Nikiphorou E. A systematic literature review to inform the 2025 EULAR recommendations for management of Polymyalgia Rheumatica and Large Vessel Vasculitis: management of disease including relapse and complications [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-systematic-literature-review-to-inform-the-2025-eular-recommendations-for-management-of-polymyalgia-rheumatica-and-large-vessel-vasculitis-management-of-disease-including-relapse-and-complications/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-systematic-literature-review-to-inform-the-2025-eular-recommendations-for-management-of-polymyalgia-rheumatica-and-large-vessel-vasculitis-management-of-disease-including-relapse-and-complications/