Session Information
Date: Monday, October 27, 2025
Title: (1612–1632) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite being considered an easy-to-diagnose and easy-to-treat condition, the optimal treatment of PMR is far from being fully elucidated. Three recently published trials have evidenced efficacy and safety of anti-IL6R agents, but their cost, as well as the high incidence and prevalence of PMR, make them unsuitable for extensive use as a first line treatment. At the same time, only a few, poorly designed, trials explored the role of cDMARDs. In addition, none of these studies adequately ruled out the presence of subclinical GCA, which, accounting for more than 20% of cases, may justify the poor response to MTX. In this regard, the aim of this prospective study was to assess whether the precocious administration of MTX, in association with a short course of GC, could be safe and effective in patients with recently diagnosed PMR, upon exclusion of subclinical GCA.
Methods: In this single arm, open label, prospective study, all patients with a recent diagnosis of PMR (< 4 weeks), fulfilling ACR/EULAR criteria with US were included. Patients with Southend pre-test score > 12 and OGUS > 1 were excluded beforehand. As a control group, an age and sex matched historical cohort of PMR treated with a monotherapy of GCs for at least 3 months from diagnosis was included.At baseline, all patients were treated with 25 mg of PDN and 0,2 mg/kg/weekly MTX; PDN was gradually tapered to 0 within 20 weeks and, in case of flare, re-increased to the minimum effective dosage.The primary endpoint of the study was PDN-free remission at 24 weeks. Secondary endpoints were sustained remission at 52 weeks (defined as clinical remission, absence of flares, CRP normalization), number of flares, PDN dosage, CRP and ESR at 24 and 52 weeks compared to historical cohort.
Results: Forty-one patients were prospectively included, while 63, age and sex matched, were included in the retrospective cohort.In the MTX-cohort, at 6-months, 58% of patients were in steroid-free remission and therefore met the primary endpoint of the study. Eight, yet without any sign or symptom of PMR, were still taking a mean daily dosage of 3,88 mg of PDN.Overall, a significant reduction of both CRP and ESR was observed. Similarly, mean daily PDN dosage dropped to 3,42 ± 3,3 mg. Seven patients (17,5%) suffered from disease flares, requiring a temporary increase of PDN dosage. Thirty-two patients, upon the exclusion of those who dropped out due to the discontinuation of MTX and/or due to the administration of biological DMARDs, reached 52-weeks phase of the study and 59% of them maintained GCs-free remission.A significantly higher number of relapses were reported within the first 12 months in the retrospective cohort (p = 0,036), in which up to 49 patients (77,8%) were still taking PDN, (4 > 7,5 mg).
Conclusion: In conclusion, upon a subclinical GCA is excluded, MTX, administered at adequate dosages, is a suitable option for the treatment of PMR, allowing a precocious discontinuation of oral GCs. The superiority of our findings can be explained by a higher dosage of MTX employed and by the exclusion of subclinical GCA, which is known to be associated with a higher relapse ratio. In this case, precocious administration of anti-IL6R agents should be warranted.
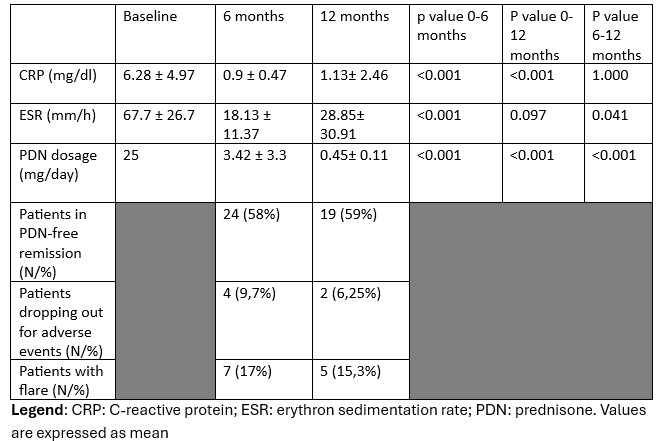 Values at baseline and during follow-up
Values at baseline and during follow-up
To cite this abstract in AMA style:
Conticini E, Grazzini S, Truscelli R, Falsetti P, Cantarini L, Frediani B. Short-Course Prednisone and Methotrexate in Polymyalgia Rheumatica: A Potential Therapeutic Approach [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/short-course-prednisone-and-methotrexate-in-polymyalgia-rheumatica-a-potential-therapeutic-approach/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-course-prednisone-and-methotrexate-in-polymyalgia-rheumatica-a-potential-therapeutic-approach/
