Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Granulomatosis with polyangiitis (GPA) is an anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis that affects all levels of the respiratory tract. Tracheobronchial stenosis (TBS) occurs in 8–23% of patients with GPA and may cause life-threatening airway compromise requiring repeated tracheal dilation. Despite advances in GPA treatment, TBS remains difficult to manage, with frequent relapses and high procedural burden. The objective of this study was to evaluate the relationship between observed immunosuppressant use and tracheal dilation frequency in patients with TBS-GPA.
Methods: We queried our electronic health record for patients with GPA and TBS evaluated at Johns Hopkins Medical Institute between January 2003 and September 2024. Diagnoses were confirmed by retrospective chart review. Baseline demographic and clinical characteristics, immunosuppressant exposure, and tracheal dilation procedure dates were extracted. We performed longitudinal analyses using annual dilation count as our outcome measure, and time-varying binary indicators of immunosuppressant use as categorical predictor variables. Data from an example patient is depicted in Figure 1. A multivariate mixed effects Poisson regression model was used to assess the association between immunosuppressant exposures (rituximab, cyclophosphamide, methotrexate, azathioprine, leflunomide, and mycophenolate) and annual tracheal dilation count, adjusting for age, sex, years since TBS diagnosis, ANCA status (positive vs. negative), and GPA disease severity (non-severe vs. severe). A random intercept term was included to account for patient-level variability in dilation frequency.
Results: A total of 52 patients with tracheobronchial GPA were included in the analysis, with a mean follow-up duration of 9.9 years. Baseline clinical characteristics and immunosuppressant exposures are summarized in Table 1. In the adjusted mixed-effects Poisson regression model, patient-years on leflunomide were associated with a 68% lower incidence of tracheal dilations compared to periods off leflunomide (IRR 0.32, p < 0.001), while patient-years on rituximab were associated with a 40% lower incidence compared to periods off rituximab (IRR 0.57, p = 0.021) (Table 2). No statistically significant associations were observed for methotrexate, azathioprine, cyclophosphamide, or mycophenolate. Among other tested covariates, time since TBS diagnosis and age over 40 were also associated with lower dilation frequency (Table 2).
Conclusion: Leflunomide and rituximab (but not methotrexate or azathioprine) were associated with significantly fewer tracheal dilations in patients with tracheobronchial GPA. Older age and longer time since diagnosis were also associated with reduced dilation frequency.
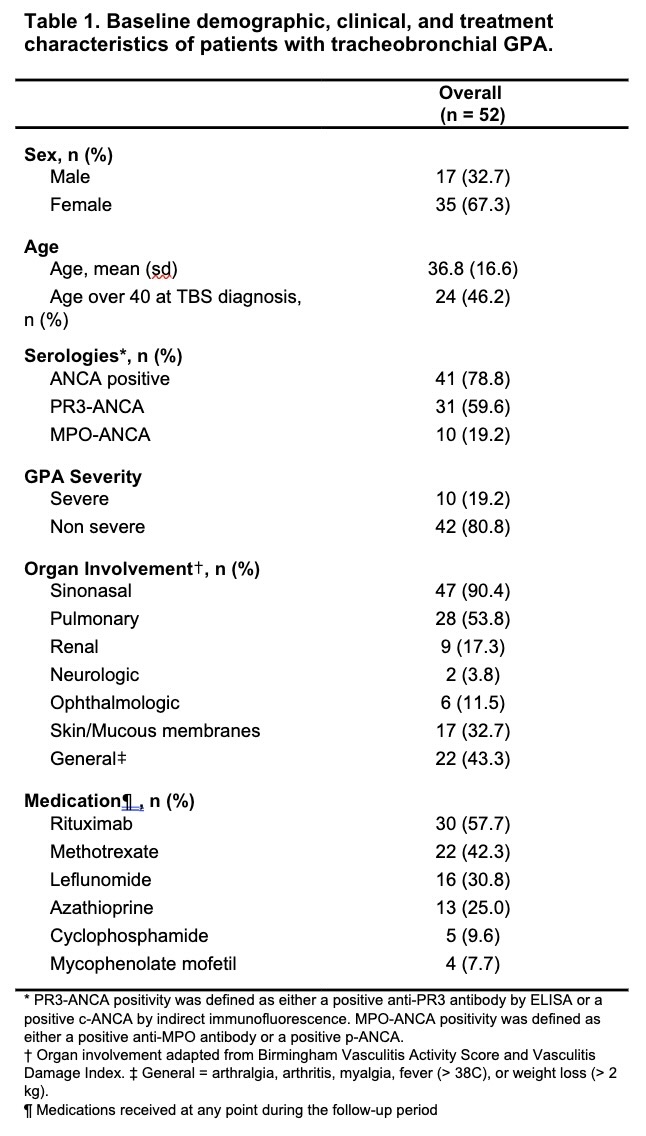 Table 1. Baseline demographic, clinical, and treatment characteristics of patients with tracheobronchial GPA.
Table 1. Baseline demographic, clinical, and treatment characteristics of patients with tracheobronchial GPA.
.jpg) Table 2. Incidence rate ratios estimated from univariate models and multivariate mixed effects Poisson regression model of tracheal dilation incidence
Table 2. Incidence rate ratios estimated from univariate models and multivariate mixed effects Poisson regression model of tracheal dilation incidence
.jpg) Figure 1. Example patient’s annual dilation counts over time with corresponding immunosuppressant exposure data.
Figure 1. Example patient’s annual dilation counts over time with corresponding immunosuppressant exposure data.
To cite this abstract in AMA style:
Denvir B, Hillel A, Shah A, Kim J, Seo P, Antiochos B. GPA-Associated Tracheobronchial Stenosis: Immunosuppressant Use and Dilation Frequency [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/gpa-associated-tracheobronchial-stenosis-immunosuppressant-use-and-dilation-frequency/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gpa-associated-tracheobronchial-stenosis-immunosuppressant-use-and-dilation-frequency/
