Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anifrolumab (ANI) is a human monoclonal antibody targeting the type I interferon receptor subunit 1 (IFNAR1), blocking interferon activity and reducing disease activity in systemic lupus erythematosus (SLE). Real-world evidence is essential to understand its use and outcomes. This study aimed to describe the clinical profiles of SLE patients treated with anifrolumab for ≥3 months at the Toronto Lupus Program and to assess its efficacy and safety.
Methods: We included patients from the University of Toronto Lupus Cohort who met the 2019 EULAR/ACR classification criteria and had received anifrolumab for ≥3 months. Demographics, clinical and lab data, prior and concomitant therapies, and disease activity indices (SLEDAI-2K, CLASI, and PGA) were recorded at baseline and during follow-up. Time to symptom resolution, prednisone dose, infections, and treatment discontinuation were also assessed.
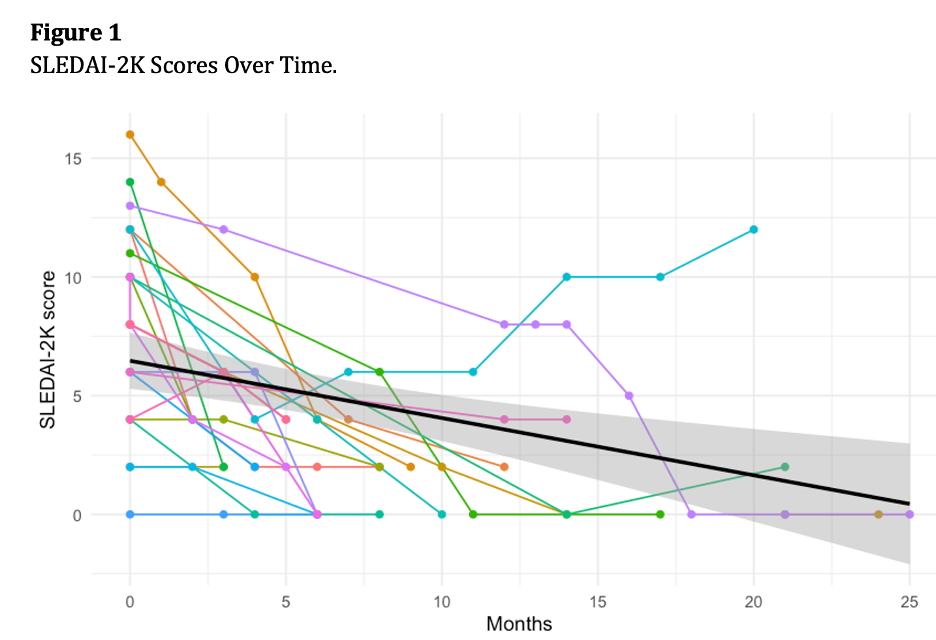
Results: Twenty-three patients (73.9% female) with a mean age of 40.4 years and mean SLE duration of 12.7 years were included. Cumulative organ involvement at anifrolumab initiation included mucocutaneous (100%), musculoskeletal (73.9%), serositis (34.8%), hematologic (34.8%), renal (30.4%), neuropsychiatric (21.7%), and persistent fever (8.7%).The main reasons for initiating anifrolumab included skin rash (n = 20, 87.0%), musculoskeletal involvement (n = 15, 65.2%), alopecia (n = 8, 34.8%), along with the presence of concomitant hematological involvement (leukopenia) and shrinking lung syndrome. Mean follow-up was 8.4 months.All patients were on prednisone at initiation; 82.6% were also on hydroxychloroquine. Concomitant immunosuppressives included mycophenolate (47.8%), methotrexate (17.4%), or both (4.3%). Prednisone decreased from 13.2 mg/day to 4.0 mg/day, and 69.6% discontinued it. Immunosuppressives were reduced or stopped in 52.2%. SLEDAI-2K scores decreased significantly over time (Figure 1). Median time to rash resolution was 57 days; full symptom resolution occurred in a median of 131 days.Anifrolumab was discontinued in two patients: one after 20 months to start another biologic for lupus nephritis, and one due to refractory molluscum contagiosum after 14 months. No additional adverse events were reported.Anifrolumab demonstrated significant effectiveness in reducing disease activity and corticosteroid use in SLE, with a favorable safety profile.
Conclusion: Anifrolumab demonstrated significant effectiveness in reducing disease activity and corticosteroid use in SLE, with a favorable safety profile.
To cite this abstract in AMA style:
Carrizo Abarza V, Li Q, Semalulu T, Smith J, Mehta P, Kharouf F, Gladman D, Whitall Garcia L, Touma Z. Real-World Outcomes of Anifrolumab in Systemic Lupus Erythematosus Patients at Toronto Lupus Program [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-outcomes-of-anifrolumab-in-systemic-lupus-erythematosus-patients-at-toronto-lupus-program/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-outcomes-of-anifrolumab-in-systemic-lupus-erythematosus-patients-at-toronto-lupus-program/