Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite the evolution of various flare definitions and their inclusion in SLE clinical trials, moderate flares tend to be underestimated in trials owing to their evaluation based on a single time interval as opposed to multiple intervals. Dapirolizumab pegol (DZP) is a novel CD40L inhibitor with broad modulatory effects on SLE immunopathology;1,2 it consists of a polyethylene glycol (PEG)-conjugated antigen-binding fragment (Fab’), which lacks an Fc domain. In the phase 3 PHOENYCS GO trial (NCT04294667) of DZP in SLE, the primary endpoint, BILAG-based Composite Lupus Assessment (BICLA) response at Week (Wk) 48, was met.3 Additionally, through Wk 48, a lower proportion of patients (pts) receiving DZP plus standard of care (DZP+SOC) vs placebo (PBO)+SOC had BILAG 2004 severe flares (11.6% vs 23.4%; nominal p=0.0257).3 We report moderate flare outcomes from PHOENYCS GO and explore alternative definitions to more accurately estimate their frequency.
Methods: PHOENYCS GO was a 48-wk, randomized, double-blind, PBO-controlled trial. Pts were randomized 2:1 to intravenous DZP 24 mg/kg+SOC or PBO+SOC every 4 wks. Pre-specified analyses defined a BILAG 2004 severe flare as ≥1 BILAG 2004 Grade A due to a new/worse individual item(s), and a BILAG 2004 moderate flare as ≥2 BILAG 2004 Grade Bs due to new/worse individual items in different organ systems at the same visit. Post hoc analyses included two alternative definitions of BILAG 2004 moderate flares that allowed for ≥2 BILAG 2004 Grade Bs due to new/worse individual items in different organ systems to occur across either: (1) up to 2 consecutive visits and (2) up to 3 consecutive visits, all visits 4 wks apart (Figure 1).
Results: Baseline characteristics are presented in the Table. At Wk 48, by the pre-specified definition, 13.9% (29/208) vs 20.6% (22/107) of pts receiving DZP+SOC vs PBO+SOC, respectively, had BILAG 2004 moderate flares. By alternative definition 1, BILAG 2004 moderate flares occurred in 15.4% (32/208) vs 29.0% (31/107) of pts receiving DZP+SOC vs PBO+SOC, respectively. By alternative definition 2, BILAG 2004 moderate flares occurred in 16.8% (35/208) vs 33.6% (36/107) of pts receiving DZP+SOC vs PBO+SOC, respectively (Figure 2). Time to BILAG 2004 moderate/severe flares through Wk 48 was shorter for pts receiving DZP+SOC vs PBO+SOC by all definitions.
Conclusion: Pts treated with DZP+SOC had fewer BILAG 2004 moderate flares, by all definitions considered, vs those treated with PBO+SOC. When allowing for longer time intervals over consecutive visits to evaluate moderate flares with the alternative definitions, greater rates of BILAG 2004 moderate flares were observed, particularly for pts receiving PBO+SOC, and greater differentiation between DZP+SOC and PBO+SOC was also observed. These results confirm a need to re-evaluate how flares are captured in SLE clinical trials; the alternative definitions of flares explored may better simulate clinical practice than the native definition of BILAG 2004 moderate flares.References: 1. Powlesland AS. Annals Rheum Dis 2024;83 (suppl 1):261; 2. Cutcutache I. Arthritis Rheumatol 2023;75 (suppl 9); 3. Clowse M. Arthritis Rheumatol 2024;76 (suppl 9).
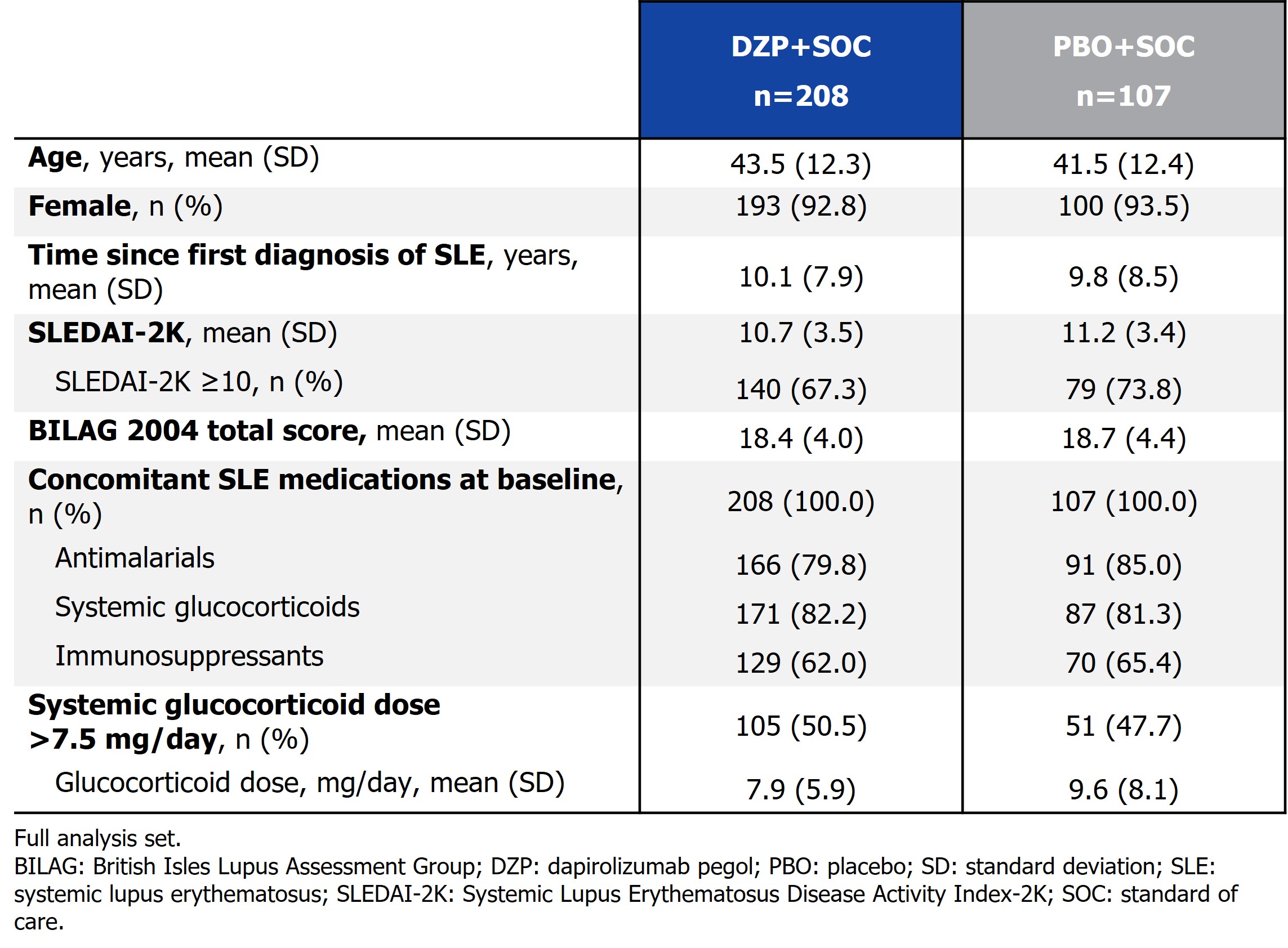 Table. Baseline demographics and disease characteristics
Table. Baseline demographics and disease characteristics
.jpg) Figure 1. Pre-specified and alternative definitions of BILAG 2004 moderate flares
Figure 1. Pre-specified and alternative definitions of BILAG 2004 moderate flares
.jpg) Figure 2. Cumulative occurrence of BILAG 2004 moderate flares across definitions
Figure 2. Cumulative occurrence of BILAG 2004 moderate flares across definitions
To cite this abstract in AMA style:
Furie R, Bertsias G, Carter L, Morand E, Mosca M, Pike M, Taieb V, Nelde A, Vital E, Stach C. Alternative Definitions of Moderate Flares That Simulate Clinical Practice in Systemic Lupus Erythematosus: Post Hoc Exploration of Moderate Flares in Patients Treated with Dapirolizumab Pegol in a 48-Week Phase 3 Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/alternative-definitions-of-moderate-flares-that-simulate-clinical-practice-in-systemic-lupus-erythematosus-post-hoc-exploration-of-moderate-flares-in-patients-treated-with-dapirolizumab-pegol-in-a-48/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/alternative-definitions-of-moderate-flares-that-simulate-clinical-practice-in-systemic-lupus-erythematosus-post-hoc-exploration-of-moderate-flares-in-patients-treated-with-dapirolizumab-pegol-in-a-48/
