Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To improve quality of life (QoL) in individuals with SLE, we developed the Whole Health Empowerment for Endotypes of Lupus (WHEEL) program—a 4-month, biweekly, virtual, SLE-specific peer support curriculum. Adapted from an existing evidenced-based group visit model and delivered by certified health coaches, WHEEL promotes self-care and engagement through education, goal setting, mindfulness, and group discussion (Fig 1). The current study conducted user testing to assess feasibility and acceptability. To optimize group dynamics and shared experience, participants were stratified into two Type 2 SLE endotypes: Intermittent (symptoms aligning with Type 1 activity) and Persistent (symptoms persisting despite Type 1 remission).
Methods: Adults with SLE (2012 SLICC or 2019 ACR/EULAR criteria) with ≥3 visits in an academic registry were screened for potential inclusion based on SLEDAI, Type 1 and Type 2 physician global assessments (PGA; 3-point visual analog scales), and the polysymptomatic distress (PSD) score. High Type 1 SLE activity was defined as clinical SLEDAI ≥4, SLEDAI ≥6, renal SLEDAI ≥4, or PGA ≥1. High Type 2 SLE activity was defined as Type 2 PGA ≥1 or PSD ≥8. Patients without high Type 2 SLE activity were not eligible.User Testing Cohorts Persistent Type 2 SLE (SLE-P): High Type 1 SLE activity at < 30% and high Type 2 SLE activity at ≥50% of visitsIntermittent Type 2 SLE (SLE-I): Fluctuating Type 2 SLE activityUser testing consisted of 8 group sessions, 2 individual health coach sessions, development of personal goals, and pre/post surveys. Feasibility and acceptability were assessed via accrual, attendance, attrition, satisfaction, and fidelity. Progress achieved on personal goals further informed feasibility. Whole Health Assessment and PROMIS surveys explored preliminary efficacy.
Results: We enrolled 5 women in each cohort (100% accrual). While race and ethnicity were similar between cohorts, the average age of the SLE-P cohort was 10 years older. Nine participants completed the program with 1 early withdrawal from SLE-P. Over 80% of all group sessions were attended, and 8 participants attended all but 1 group session. On average, participants set 3 personal goals. All participants made progress on at least 1 goal, and 7 of 9 participants achieved ≥1 personal goal.Program fidelity was high (98%). Participants reported excellent program satisfaction (96%) and provided overwhelmingly positive feedback (Table 1).Post-WHEEL, PROMIS pain intensity and pain interference scores improved in SLE-I, while PROMIS medication self-efficacy improved in SLE-P (Table 2). Both cohorts reported gains in Whole Health nutrition and movement/activity; SLE-I additionally showed improvement in rest, purpose, stress, and environment, whereas SLE-P improved in relationships.
Conclusion: The WHEEL program was developed to improve quality of life in SLE. User testing demonstrated strong accrual, retention, acceptance, fidelity, and satisfaction. Participants progressed toward personal health goals and exhibited improvement across several outcome domains. Findings support the feasibility of a multi-modal, whole health approach for SLE and are informing adaptations of the WHEEL program.
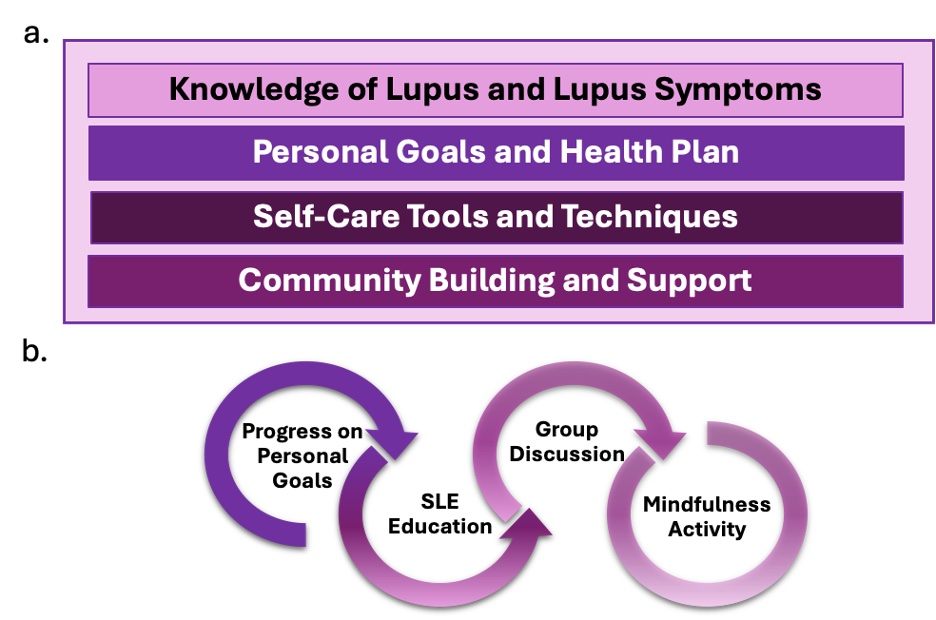 Figure 1a. Aspects of the WHEEL program. 1b. Components of each WHEEL group session
Figure 1a. Aspects of the WHEEL program. 1b. Components of each WHEEL group session
.jpg) Table 1. Anonymous feedback from User testing participants
Table 1. Anonymous feedback from User testing participants
.jpg) Table 2. Lupus Outcomes Pre and Post WHEEL intervention
Table 2. Lupus Outcomes Pre and Post WHEEL intervention
To cite this abstract in AMA style:
Rogers J, Eudy A, Drake C, Somers T, Pisetsky D, Clipper C, Snyderman R, Batsakes A, Saner L, Burshell D, Maheswaranathan M, Criscione-Schreiber L, Sadun R, Harris N, Sun K, Dunn K, Herndon J, Jacobs V, Clowse M. Whole Health Empowerment for Endotypes of Lupus (WHEEL) Program: User Testing Findings on Feasibility, Acceptability, and Opportunities for Intervention Refinement [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/whole-health-empowerment-for-endotypes-of-lupus-wheel-program-user-testing-findings-on-feasibility-acceptability-and-opportunities-for-intervention-refinement/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/whole-health-empowerment-for-endotypes-of-lupus-wheel-program-user-testing-findings-on-feasibility-acceptability-and-opportunities-for-intervention-refinement/
