Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Secukinumab is a fully human monoclonal antibody that selectively inhibits interleukin-17A, demonstrating robust and sustained efficacy in moderate to severe psoriasis (PsO) and psoriatic arthritis (PsA) with a good safety profile in long-term trials.1-3 Besides its efficacy in PsA, secukinumab may have a preventive effect in patients with PsO without clinical signs of PsA. Pooled clinical trials of secukinumab (ERASURE, FIXTURE and SCULPTURE) provide valuable data sets that can help in understanding PsA prevention with secukinumab treatment. This study aimed to explore the efficacy of secukinumab in preventing PsA in patients with PsO using pooled data from the ERASURE, FIXTURE and SCULPTURE trials and to indirectly compare these data with historical data in patients who were biologic-naïve.4
Methods: Patients with moderate to severe PsO (with no medical history of PsA at baseline) who were treated with secukinumab 300 mg through up to 5-year follow-up were included in this analysis. The incidence of PsA within the studies was assessed using exposure-adjusted incidence rates (EAIRs; incidence rate/100 patient-years [PY]). The preferred term ‘psoriatic arthropathy’ in the reported adverse events was used to capture the incident PsA. The time to develop PsA was estimated using Kaplan-Meier analysis. Efficacy was measured using Psoriasis Area and Severity Index (PASI) 90 and 100 responders (as observed data).
Results: Overall, 673 patients with active PsO without PsA were included in the analysis (mean age: 45.2 years; male: 71.8%; mean body mass index: 28.9 kg/m2; mean time since diagnosis: 16.1 years). In total, 8% (Nf54), 11.3% (Nf76), 34.5% (Nf232) and 46.2% (Nf311) patients had < 2, 2- < 5, 5- < 15 and ≥15 years of disease duration, respectively. Overall, 18.3% of patients were biologic-experienced before the start of secukinumab. All patients had a PASI score of ≥10. After 5 years of treatment and a total exposure of 1831 PY, most patients (659/673 patients; 97.9%) did not experience PsA (Fig. 1). This translates into an EAIR of 0.76 (95% CI: 0.42,1.28). An 8-year prospective observational study previously reported a yearly PsA incidence rate of 2.7/100 patients in biologic-naïve patients with PsO without PsA.4 When we indirectly compared these data with current data from pooled clinical trials, we found that incidence rate of PsA development was ~72% lower with secukinumab. At Year 5 of secukinumab treatment, 36.5% and 63.9% of patients achieved skin clearance (PASI 100) and PASI 90, respectively (Table 1).
Conclusion: These findings support the hypothesis that secukinumab may prevent PsA development in patients with PsO and suggest a potential protective effect against PsA. Additionally, secukinumab-treated patients demonstrated effective and sustained long-term control over skin clearance. References:1. Langley RG, et al. Br J Dermatol. 2023;188:198-2072. Bissonnette R, et al. J Eur Acad Dermatol Venereol. 2018;32:1507-14. 3. Sun R, et al. Dermatol Ther (Heidelb). 2024;14:729-43. 4. Eder L. Arthritis Rheumatol. 2016;68:915-23.
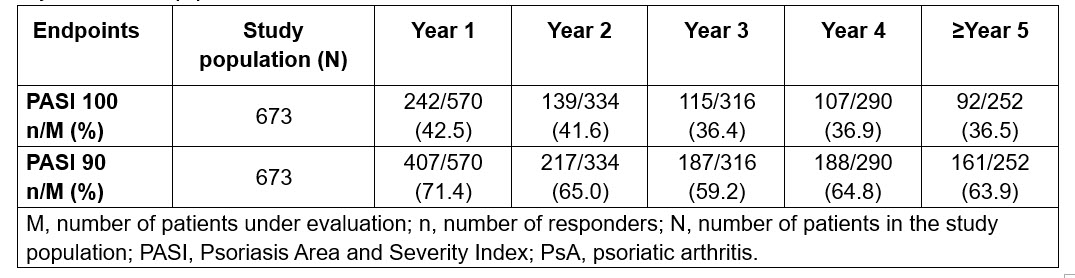 Table 1. Efficacy of secukinumab in patients with psoriasis without PsA at baseline over a 5-year follow-up period.
Table 1. Efficacy of secukinumab in patients with psoriasis without PsA at baseline over a 5-year follow-up period.
.jpg) Figure 1: Kaplan-Meier Curve for PsA development in patients with psoriasis without PsA at baseline over a 5-year follow-up period.
Figure 1: Kaplan-Meier Curve for PsA development in patients with psoriasis without PsA at baseline over a 5-year follow-up period.
To cite this abstract in AMA style:
Mrowietz U, Coates L, Thaçi D, Lebwohl M, Sigurgeirsson B, Warren R, Langley R, Clemens A, Vizcaya C, Bao W, Hoyt K, Gómez L, Bissonnette R. Long-term impact of secukinumab on the prevention of psoriatic arthritis in patients with psoriasis: a 5-year pooled analysis of the ERASURE, FIXTURE and SCULPTURE studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/long-term-impact-of-secukinumab-on-the-prevention-of-psoriatic-arthritis-in-patients-with-psoriasis-a-5-year-pooled-analysis-of-the-erasure-fixture-and-sculpture-studies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-impact-of-secukinumab-on-the-prevention-of-psoriatic-arthritis-in-patients-with-psoriasis-a-5-year-pooled-analysis-of-the-erasure-fixture-and-sculpture-studies/
