Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The potential that nonsteroidal anti-inflammatory drugs (NSAIDs) may precipitate flares of inflammatory bowel disease (IBD) has limited their use in managing joint pain in this population. We tested whether use of NSAIDs is associated with a clinically important risk of IBD-related hospitalization using a non-inferiority design.
Methods: This retrospective cohort study of IBD patients used administrative claims data from Optum’s de-identified Clinformatics Data Mart Database (2000-2022). Patients with a new NSAID prescription fill (“exposed”) were matched to those without an NSAID fill during the study period (“unexposed”) by age and calendar year when IBD criteria were met (Figure 1). The index date for exposed patients was date of first NSAID fill; unexposed patients were assigned a matched index date based on time from meeting IBD criteria. A propensity score was calculated to balance covariates across exposure groups. The primary outcome was IBD-related hospitalization, defined as any hospitalization >1 day duration with an IBD diagnosis code as the primary or secondary discharge code. Secondary outcomes included all-cause hospitalization and gastrointestinal surgery, defined by Current Procedural Terminology codes. Propensity score-based inverse probability of treatment weighted (IPTW) Cox proportional hazards models evaluated the association between NSAID exposure and time to each primary and secondary outcome across IBD subtypes. A hazard ratio (HR) of 1.2 was set as the pre-specified non-inferiority margin based on discussions with IBD content experts on acceptable risk.
Results: Among 269,304 patients with IBD, 29.7% were NSAID exposed (Table 1). In the IPTW-weighted Cox model, NSAID exposure was associated with a small increase in IBD-related hospitalization in the overall IBD cohort (HR 1.08, 95% CI 1.05-1.11; Figure 2) but demonstrated non-inferiority (upper limit of confidence interval < 1.20). Exposed patients experienced an adjusted 0.06 IBD-related hospitalizations/person-year versus 0.05 events/person-year in the unexposed group (absolute risk difference 0.007, number needed to harm 137). No significant increase in IBD-related hospitalization was seen in the ulcerative colitis subgroup (HR 0.96, 95% CI 0.91-1.01), but there was a significant increase in those with Crohn’s disease (HR 1.16, 95% CI 1.11-1.21), with non-inferiority not met. There were significantly higher rates of gastrointestinal surgery (overall HR 1.23, 95% CI 1.19-1.28) and all-cause hospitalization (overall HR 1.30, 95% CI 1.28-1.32) with neither meeting non-inferiority.
Conclusion: Prescription NSAID exposure was associated with a numerically small risk of IBD-related hospitalization below the pre-specified non-inferiority threshold. Risk was not seen in patients with ulcerative colitis, while small magnitude risks were present in those with Crohn’s disease. These results challenge the current paradigm of avoiding NSAIDs in all patients with IBD and suggest that the benefits of NSAIDs may outweigh small possible risks for many patients with significant joint disease.
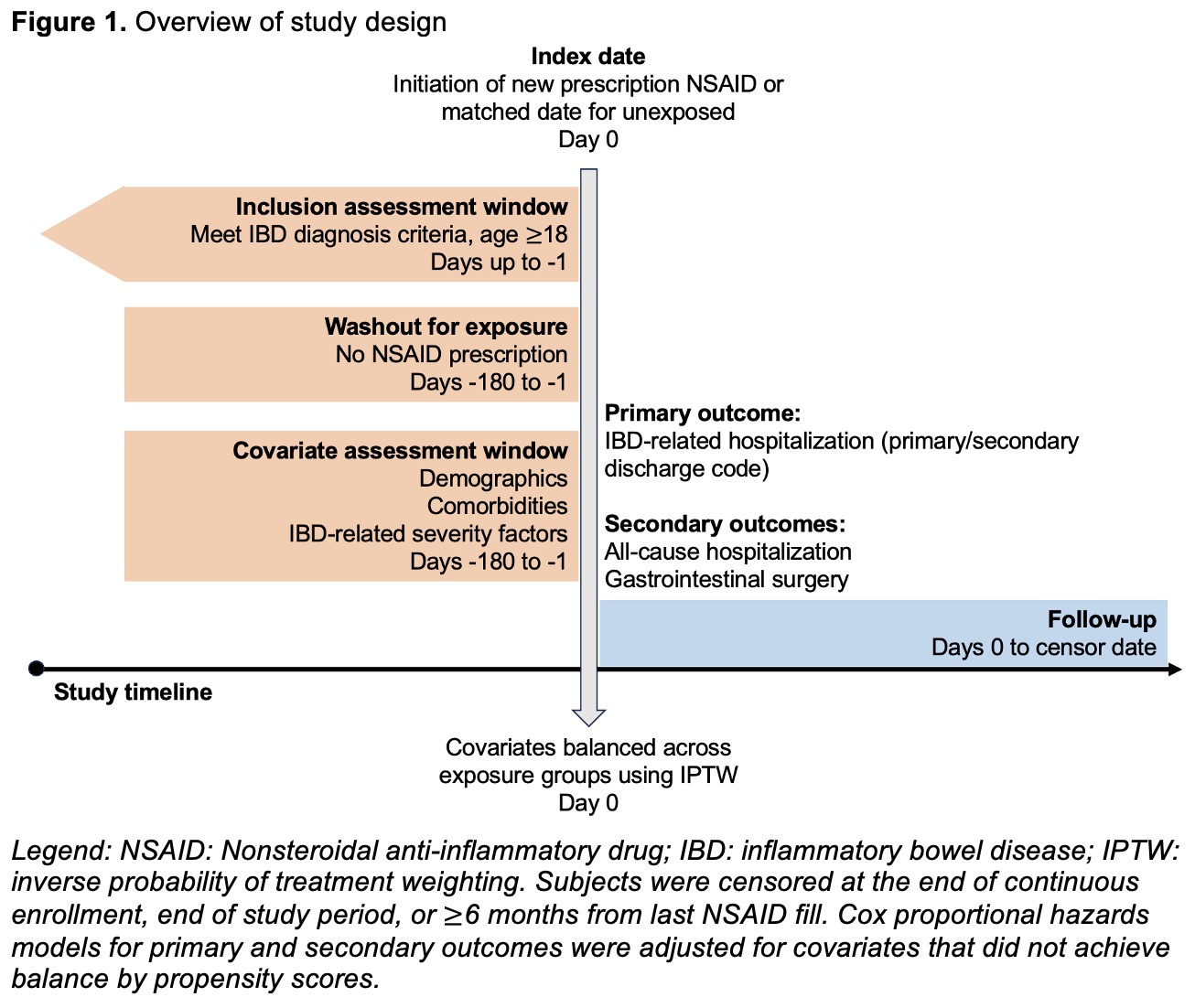 Figure 1. Overview of study design
Figure 1. Overview of study design
.jpg) Table 1. Unweighted cohort characteristics stratified by NSAID exposure
Table 1. Unweighted cohort characteristics stratified by NSAID exposure
.jpg) Figure 2. Association between prescription NSAID exposure and time to IBD-related hospitalization, all-cause hospitalization, and gastrointestinal surgery across IBD subtypes.
Figure 2. Association between prescription NSAID exposure and time to IBD-related hospitalization, all-cause hospitalization, and gastrointestinal surgery across IBD subtypes.
To cite this abstract in AMA style:
Mayer A, Xiao R, Bewtra M, George M, Weiss P. Safety of Prescription NSAIDs in Adults with IBD: Data from a Large Administrative Claims Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-of-prescription-nsaids-in-adults-with-ibd-data-from-a-large-administrative-claims-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-prescription-nsaids-in-adults-with-ibd-data-from-a-large-administrative-claims-cohort/
