Session Information
Date: Monday, October 27, 2025
Title: (1221–1247) Pain in Rheumatic Disease Including Fibromyalgia Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Fibromyalgia is a chronic condition marked by widespread pain that can significantly reduce a patient’s quality of life. While low-dose naltrexone (LDN) has emerged as a potential treatment option, its use is not yet well supported by robust evidence, particularly from randomized controlled trials. (RCTs). This study evaluates the effectiveness and safety of LDN in managing symptoms of fibromyalgia.
Methods: This meta-analysis evaluated the safety and efficacy of LDN in fibromyalgia by systematically reviewing RCTs published through December 2024. Study selection followed PRISMA guideline. Data was analyzed using randome-effect model to calculate the pooled odds ratios (ORs) and Standardized mean difference (SMDs) for the measured outcomes.
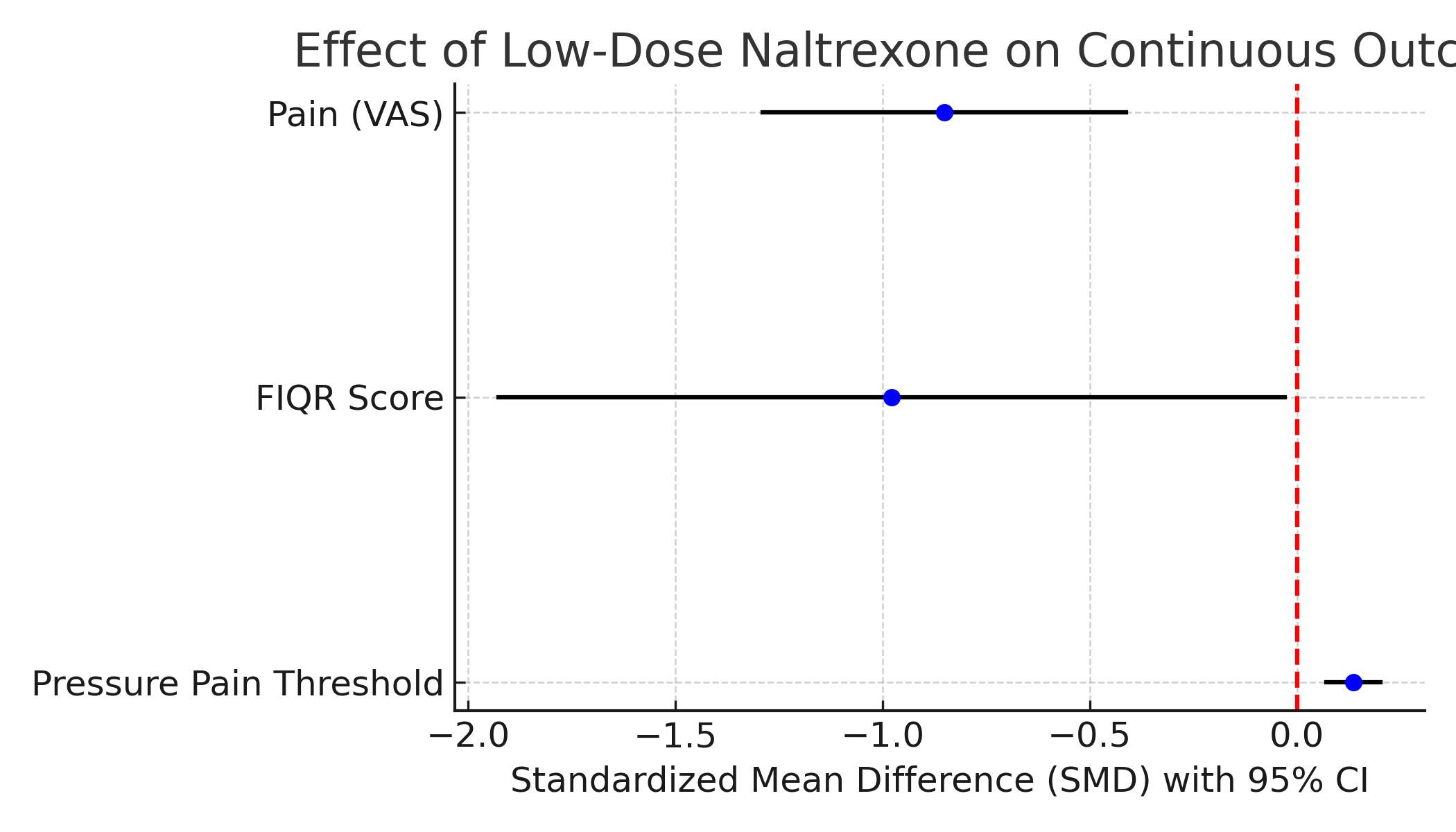
Results: A total of five RCTs were included. LDN significantly reduced pain scores compared to placebo, with a pooled SMD of –0.851 (95% CI: –1.290 to –0.412). LDN also improved functional status as measured by the Fibromyalgia Impact Questionnaire Revised (FIQR), with SMD of –0.978 (95% CI: –1.926 to –0.030). Pressure pain threshold showed a small but statistically significant increase in the LDN group (SMD: 0.136; 95% CI: 0.071 to 0.202). Regarding safety, LDN was associated with a higher odds of vivid dreams (OR: 2.17; 95% CI: 1.17 to 4.02), but no significant increase in the risk of headaches (OR: 1.41; 95% CI: 0.76 to 2.60) or nausea (OR: 1.40; 95% CI: 0.81 to 2.43). Heterogeneity ranged from low to moderate across outcomes, and no major safety concerns were identified.
Conclusion: The findings of our analysis indicate that LDN may effectively reduce pain symptoms in patient with fibromyalgia while maintaining the favorable safety profile.
To cite this abstract in AMA style:
Rizwan R, Grewal S, Banda S, Hazique M. Efficacy And Safety of Low-dose Naltrexone In Fibromyalgia: An Updated Systematic Review And Meta-Analysis of Randomized Controlled Trials (RCTs) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-low-dose-naltrexone-in-fibromyalgia-an-updated-systematic-review-and-meta-analysis-of-randomized-controlled-trials-rcts/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-low-dose-naltrexone-in-fibromyalgia-an-updated-systematic-review-and-meta-analysis-of-randomized-controlled-trials-rcts/

.jpg)