Session Information
Date: Monday, October 27, 2025
Title: (1147–1190) Miscellaneous Rheumatic & Inflammatory Diseases Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Familial Mediterranean Fever (FMF) is an autoinflammatory disease mainly treated with colchicine. Anakinra, an interleukin-1 receptor antagonist, is used for colchicine-resistant cases. However, data on the safety of Anakinra during pregnancy is limited. This study aims to evaluate pregnancy outcomes of FMF patients who received at least one dose of Anakinra during pregnancy.
Methods: A retrospective single-center study was conducted to analyze clinical data and pregnancy outcomes of FMF patients who received Anakinra during pregnancy.
Results: This study included data from 27 pregnancies involving 19 mothers. The average maternal age at pregnancy was 31.5 ± 3.95 years. The most common genotype among mothers was homozygous M694V, observed in %72.2 (13/18) of cases. Amyloidosis was present in %15.8 (3/19) of mothers. In %51.9 (14/27) of the pregnancies, mothers were on Anakinra treatment prior to pregnancy, while in the remaining pregnancies Anakinra treatment was started during pregnancy. Out of the 27 pregnancies, 26 resulted in delivery, and one is still ongoing at 15 weeks of gestation. Among the terminated pregnancies, 26 babies were born, one of whom was stillborn. The frequency of cesarean section was 80.8% (21/26), and the rate of preterm birth was 34.6% (9/26), with an average gestational age at birth of 36.6 ± 3.3 weeks. Assisted reproduction techniques were utilized in %30.4 (7/23) of the pregnancies. The most common maternal complication during pregnancy was preeclampsia (5/23) followed by bleeding (2/23), hypertension (2/23), gestational diabetes (1/23), and cerebrovascular event (1/23). Intrauterine growth restriction was reported in %18.5 (5/27) of the pregnancies. The rate of neonatal ICU hospitalization was %16.7 (4/24) among babies. It was reported that %34.8 (8/23) of the babies were small for gestational age. Mean weight, length, and head circumference of the babies were 2.8 ± 0.76 kg, 46.5 ± 6.2 cm, and 33.8 ± 3.8 cm, respectively. The average Apgar scores at first and fifth minutes were 7.8 ± 1.4 and 9.0 ± 1.1, respectively. The average current age of the children is 75.3 ± 45.9 months, with a mean weight of 26.7 ± 11.4 kg and mean height of 116.4 ± 22.8 cm. Among these children, the most common chronic condition was allergic conditions (4/9), followed by growth hormone deficiency (1/9), PFAPA (1/9), torticollis (1/9), and a heart murmur (1/9).
Conclusion: Although complications such as preeclampsia and intrauterine growth restriction were observed, no lasting sequelae were reported in the children, and the majority of pregnancies resulted in live births with favorable outcomes. Anakinra appears to be safe during pregnancy, with no major teratogenic effects identified. However, long-term follow-up is warranted to fully assess potential outcomes.
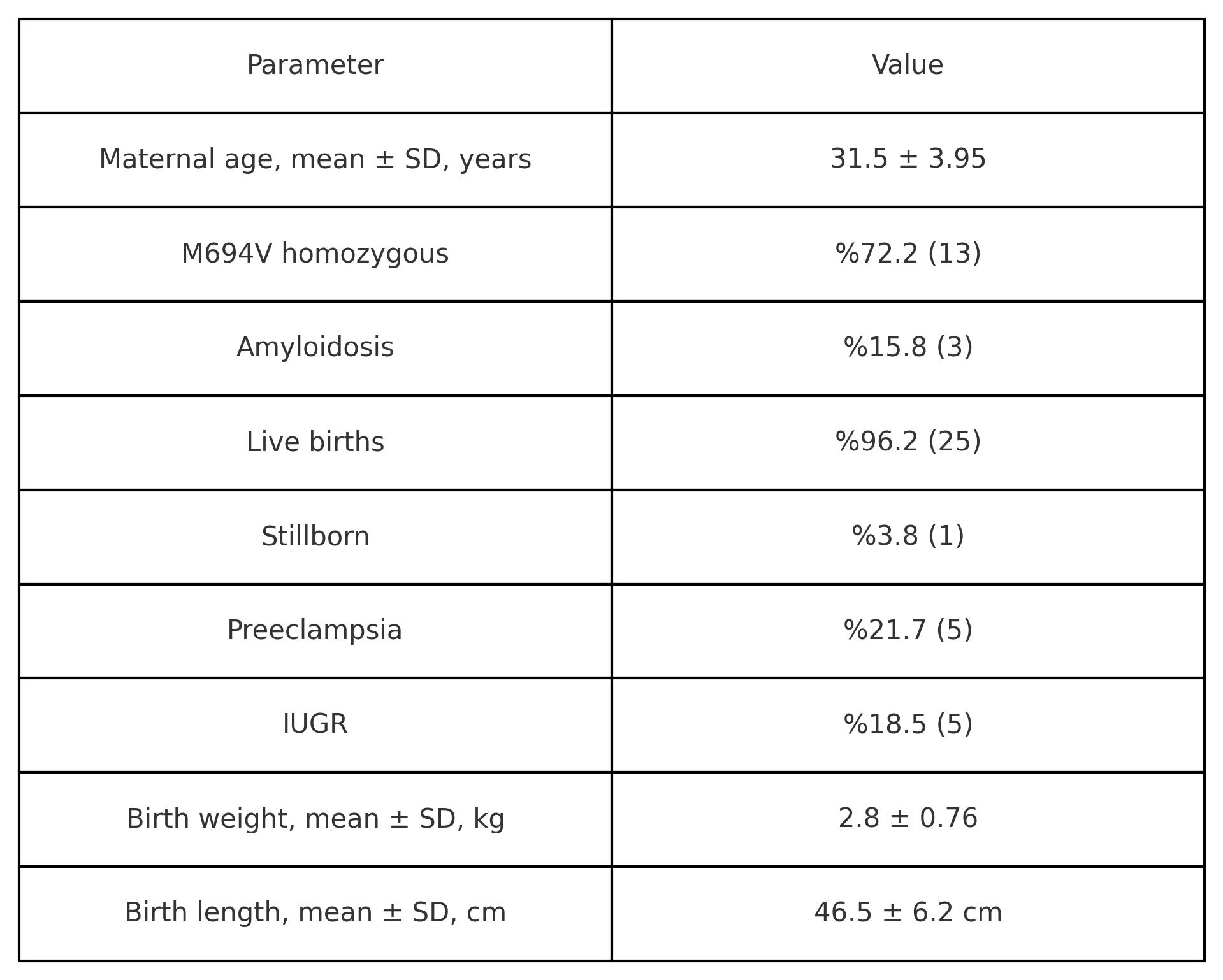 Maternal and Neonatal Characteristics of the Patient Population
Maternal and Neonatal Characteristics of the Patient Population
To cite this abstract in AMA style:
Ergezen B, Kilinc O, Egeli B, Kizilkaya O, parlar K, Azman E, ozdogan H, Ugurlu s. Pregnancy Outcomes in Women with Familial Mediterranean Fever Treated with Anakinra: A Retrospective Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/pregnancy-outcomes-in-women-with-familial-mediterranean-fever-treated-with-anakinra-a-retrospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-outcomes-in-women-with-familial-mediterranean-fever-treated-with-anakinra-a-retrospective-study/
