Session Information
Date: Monday, October 27, 2025
Title: (1088–1122) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) used for cancer therapy can have varied toxicities among which pneumonitis (ICI-pneumonitis) is a rare (< 5%) but often fatal complication. There is scant data to guide second-line therapy for ICI-pneumonitis if systemic steroids are insufficient. We sought to evaluate and compare the effectiveness of key second-line immunosuppressive agents in achieving ICI-pneumonitis resolution.
Methods: For this retrospective observational study, we identified patients with ICI-pneumonitis whose symptoms were refractory or recurrent after systemic steroid treatment and who received a second line agent. We collected data regarding cancer type, ICI type, pneumonitis phenotype and grade, pulmonary comorbidities, steroid details, secondary immunosuppression and outcomes including time to pneumonitis resolution, overall survival, and progression free survival. Count data, t-tests, and chi-square tests were used when appropriate. Cox proportional hazards regression was used for survival data.
Results: Of 1156 patients with ICI-pneumonitis, 13 ICI-pneumonitis patients with second-line treatment (mean age 60.5 (SD 16.5), 8 (61.5%) males) were included in the study. 6 (46.1%) were previous or current smokers, and 9 (69.2%) had pulmonary involvement of their malignancy. 8 (61.5%) patients received chest radiation. All patients had received anti-PD1 monotherapy. Second line immunosuppressive agents included tocilizumab (6 patients), infliximab (4 patients), mycophenolate mofetil (MMF) (4 patients), and Intravenous immunoglobulin (IVIg) (2 patients). Among these, 2 were treated with tocilizumab after failing infliximab or IVIG; one received MMF after failing infliximab. Cancer progression occurred in 2 (50%) MMF, 1 (25%) infliximab, 1 (16.7%) tocilizumab, and 0% patients receiving IVIg. Pneumonitis resolution or sustained improvement occurred most frequently with tocilizumab (4 (66.7%)) compared to MMF (1 (25%), IVIg (0%), or infliximab (0%). The hazard of the combined outcome of progression or death was lower in patients receiving tocilizumab compared to those without; however did not reach statistical significance (Figure 1; HR 0.56, 95% CI 0.16-2.01).
Conclusion: This study, while underpowered, demonstrates that tocilizumab is not associated with worse outcomes and may be associated with improved survival in patients with ICI-pneumonitis. Future studies should prospectively investigate this finding in larger cohorts.
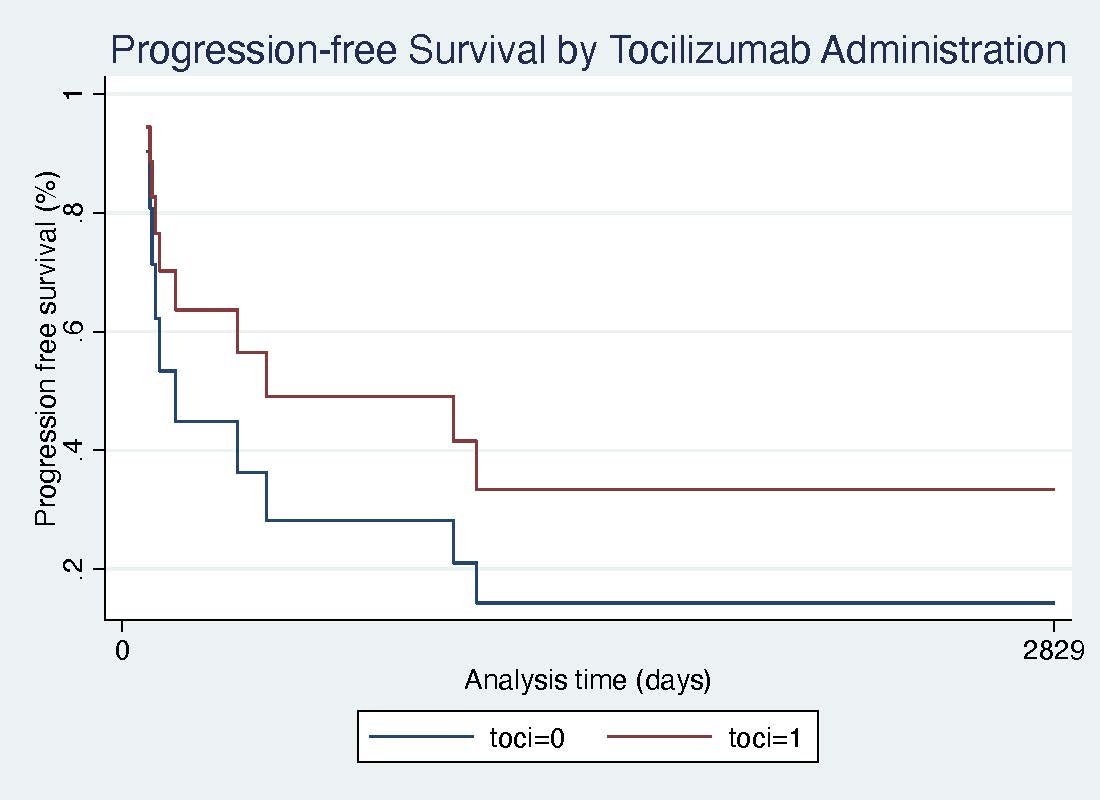 Figure 1. Progression-free survival in refractory or recurrent ICI-pneumonitis patients who received tocilizumab (toci=1, red line) compared to patients who did not receive tocilizumab (toci=0, blue line).
Figure 1. Progression-free survival in refractory or recurrent ICI-pneumonitis patients who received tocilizumab (toci=1, red line) compared to patients who did not receive tocilizumab (toci=0, blue line).
To cite this abstract in AMA style:
Sun S, Lee C, Reid P. Second-line Therapy for Immune-checkpoint Inhibitor-induced Pneumonitis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/second-line-therapy-for-immune-checkpoint-inhibitor-induced-pneumonitis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/second-line-therapy-for-immune-checkpoint-inhibitor-induced-pneumonitis/
