Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Biologic therapies have transformed rheumatologic disease management over the past two decades. As utilization of these high-cost medications increases and additional biologic and biosimilar therapies enter the market, understanding their financial and economic impact on the healthcare system is critical. Given rising Medicare part D drug spending, insights from longitudinal cost data can inform future policy and highlight areas for cost containment. This study leverages national Medicare Part D data from 2013 to 2022 to evaluate the cost of biologics prescribed for rheumatologic conditions with focus on the influence of market competition.
Methods: This is a retrospective analysis of publicly available Medicare Part D Prescription Drug Event Data from 2013 to 2022 for Xeljanz, Olumiant, Rinvoq, Actemra, Kevzara, and Orencia. For each medication, the total number of prescribers, claims, and 30-day prescriptions as well as the total cost per year were captured. Claims attributed to non-rheumatologists were excluded. All cost values were inflation-adjusted to 2022 US dollars using the Medical Care Consumer Price Index. Trends were analyzed to evaluate changes in utilization and cost over time.
Results: The number of and cost per 30-day prescriptions increased annually, outpacing inflation, for all biologics from 2013-2022 (Figure 1). While 30-day prescription costs increased overtime, the annual percent increase declined during the study period for Xeljanz, Actemra, and Orencia (Figure 2). While additional JAK inhibitors and an IL-6 inhibitor received FDA approval and entered the market during this period, ANOVA showed no significant difference in this decline among these three drugs (F(2, 24) = 0.094, p = 0.91), including Orencia which lacks a direct market competitor. A strong correlation was also observed between drug cost (30-day prescription cost) and utilization (annual number of 30-day prescriptions) as seen in Figure 3 (r = 0.96 (Xeljanz), 0.76 (Olumiant), 0.82 (Rinvoq), 0.86 (Actemra), 0.96 (Kevzara), 0.91 (Orencia)).
Conclusion: This analysis demonstrates that the cost of biologics continues to increase, surpassing inflation. While the rate of increase is declining, these results suggest that market competition alone may not be a sufficient mechanism for controlling drug prices. Notably, the rate of decline in annual cost increases was similar across Xeljanz, Actemra, and Orencia—despite Orencia lacking a direct market competitor during the study period. This finding challenges the assumption that the entry of new therapies, including biosimilars, will lead to substantial price reductions. As biosimilar availability expands, their ability to generate meaningful cost savings may be more limited than anticipated. These results highlight the need for broader policy interventions and innovative reimbursement models to effectively address the growing burden of drug spending in rheumatology.
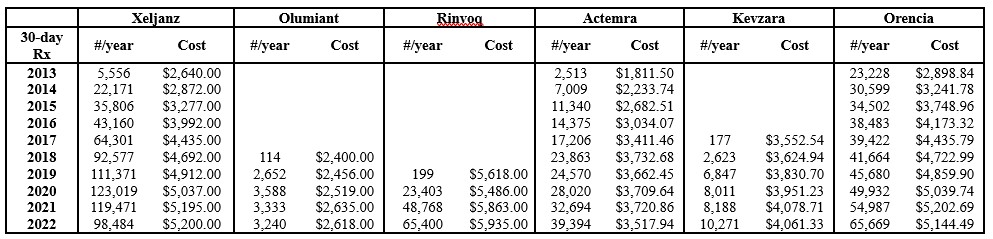 Figure 1. Annual number of and cost per 30-day prescription for selected biologics for rheumatologic indications 2013-2022. Cost is adjusted for inflation using Medical Care Consumer Price Index. As Olumiant, Rinvoq, and Kevzara entered the market during the study period, data reporting for each drug begins in the year it received FDA approval.
Figure 1. Annual number of and cost per 30-day prescription for selected biologics for rheumatologic indications 2013-2022. Cost is adjusted for inflation using Medical Care Consumer Price Index. As Olumiant, Rinvoq, and Kevzara entered the market during the study period, data reporting for each drug begins in the year it received FDA approval.
.jpg) Figure 2. Comparison of the annual percent increase in cost of 30-day prescription for Xeljanz, Actemra, and Orencia 2014-2022. Triangles denote years in which a same-class drug came to market. The square denotes the year of the introduction of Xeljanz’s black box warning.
Figure 2. Comparison of the annual percent increase in cost of 30-day prescription for Xeljanz, Actemra, and Orencia 2014-2022. Triangles denote years in which a same-class drug came to market. The square denotes the year of the introduction of Xeljanz’s black box warning.
.jpg) Figure 3. Correlation of annual number of 30-day prescriptions and cost per 30-day prescription for (A) Rinoq, (B) Actemra, and (C) Orencia.
Figure 3. Correlation of annual number of 30-day prescriptions and cost per 30-day prescription for (A) Rinoq, (B) Actemra, and (C) Orencia.
To cite this abstract in AMA style:
Koenig T. Trends in Cost and Utilization of Biologic Medications for Rheumatologic Conditions: A Medicare Claims Study (2013–2022) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/trends-in-cost-and-utilization-of-biologic-medications-for-rheumatologic-conditions-a-medicare-claims-study-2013-2022/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/trends-in-cost-and-utilization-of-biologic-medications-for-rheumatologic-conditions-a-medicare-claims-study-2013-2022/
