Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multiorgan organ damage, and early mortality. Anifrolumab (ANI), a human monoclonal antibody targeting the type I interferon receptor, is approved for the treatment of moderate to severe SLE. However, its efficacy and safety profile for cutaneous lupus erythematosus (CLE) is limited. Our aim was to assess the efficacy and safety of ANI in subgroups of SLE and CLE.
Methods: Patients with SLE were identified from each hospital’s electronic database between April 2015 and April 2025 in five centers after receiving IRB approval. Those aged >18 years who were receiving ANI every 4 weeks were included in the study. These patients were categorized into four subgroups: Total cohort, SLE only, SLE and CLE, and CLE (subacute and chronic) only. Data collected encompassed sociodemographic details, clinical features, SLE organ involvement, laboratory findings, previous and current medications along with their adverse effect profiles, and disease activity indices. Serial measurements of C3, C4, dsDNA, SLEDAI-2k, CLASI-A, and CLASI-D, as well as adverse effects related to ANI, were tracked over a 12-month period. Descriptive statistics were used for data analysis.
Results: The total cohort (n = 63) was predominantly female (88.9%) and of Arab ethnicity (66.7%) with a mean SLE disease duration of 87.3 ± 87.8 months. Mucocutaneous involvement was the most common disease manifestation (74.6%), followed by musculoskeletal (73%) and hematological (61.9%) domains. The mean age at ANI initiation was 37.9 ± 11 years and the mean duration on ANI was 9.7 ± 5.7 months. The most reported adverse event reported was upper respiratory tract infection (URTI) (12.7%), with one patient report of both URTI and gastroenteritis (Table 1). The SLE subgroup had the longest disease duration at 100.3 ± 89.9 months and had the highest previous use of prednisolone (87.5%) and the highest prevalence of adverse effects from prior therapy (37.5%). The SLE and CLE subgroup commonly had mucocutaneous (100%) and musculoskeletal involvement (76.2%), with a higher anti-Sm (59.5%) and SSA positivity (61.9%) compared to the SLE only group. They received immunosuppressive agents such as MMF (38.1%) and belimumab (30.9%). Mean duration on anifrolumab was 7.6 ± 5.5 months and 14.3% reported URTI as an adverse event. The CLE subgroup consisted mainly of females (80%). Organ involvement was limited to the skin, with chronic cutaneous lupus being most common (80%), followed by subacute cutaneous lupus (20%). Serologies were less frequently positive. Prior immunosuppressive use was limited. The mean age of ANI initiation was 32 ± 9.8 years and remained on ANI for 9.8 ± 5.5 months with no adverse events reported. Over the 12-month follow up, disease activity scores and laboratory investigations across all subgroups showed numerical improvement (Figure 1).
Conclusion: Anifrolumab demonstrated a favorable safety and tolerability profile across both SLE and CLE in a multicenter setting. These findings support the expansion of the role of ANI in CLE and highlight the need for further studies to understand its utility across the lupus spectrum.
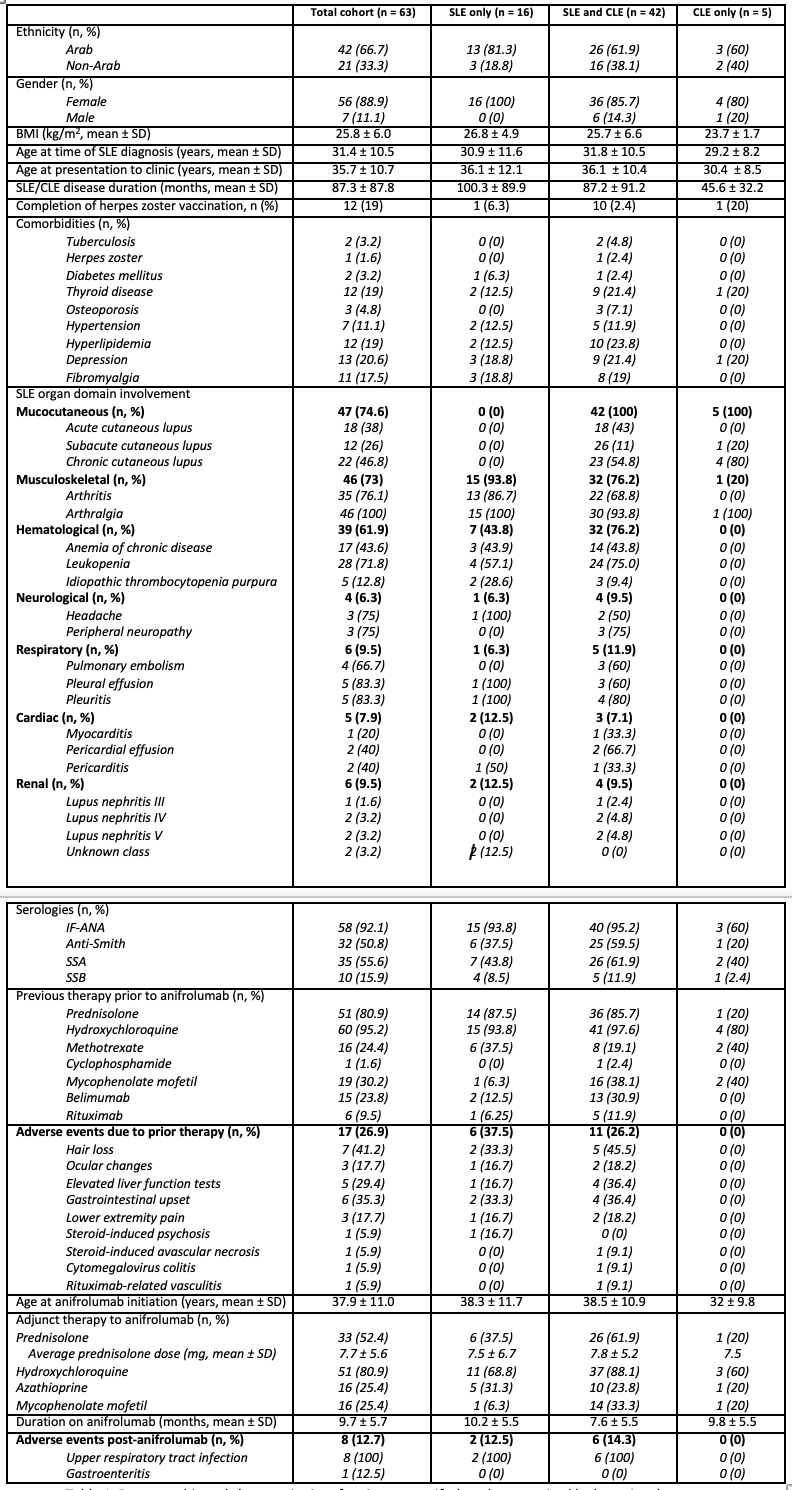 Demographic and characteristics of patients on anifrolumab categorized by lupus involvement.
Demographic and characteristics of patients on anifrolumab categorized by lupus involvement.
.jpg) Changes in disease activity scores and levels over a 12-month period on anifrolumab.
Changes in disease activity scores and levels over a 12-month period on anifrolumab.
To cite this abstract in AMA style:
Namas R, Al Qassimi S, Abdou R, Alameri J, Alblooshi R, Abdulla F, Ghosn M, Malik A, Almaashari R, Salvo F, Abuzakouk M, Al Ansari A, Rifaai H, Almarzooqi A, Mubashir A, Aldhaheri A, Attar S, Elarabi M. Real World Comparative Use of Anifrolumab in Systemic Lupus Erythematosus and Cutaneous Lupus Erythematosus: A Multicenter Cohort Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-comparative-use-of-anifrolumab-in-systemic-lupus-erythematosus-and-cutaneous-lupus-erythematosus-a-multicenter-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-comparative-use-of-anifrolumab-in-systemic-lupus-erythematosus-and-cutaneous-lupus-erythematosus-a-multicenter-cohort-study/
