Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Dapirolizumab pegol (DZP) is a novel CD40L inhibitor with broad modulatory effects on SLE immunopathology;1,2 it consists of a polyethylene glycol (PEG)-conjugated antigen-binding fragment (Fab’), which lacks an Fc domain. In the phase 3 PHOENYCS GO trial (NCT04294667), the primary endpoint was met; DZP plus standard of care (DZP+SOC) resulted in significantly higher rates of BILAG-based Composite Lupus Assessment (BICLA) response vs placebo (PBO)+SOC at Week (Wk) 48.3 Remission (based on Definition of Remission in SLE [DORIS]) and low disease activity (based on Lupus Low Disease Activity State [LLDAS]) are recommended treatment targets in SLE,4 and achievement of both DORIS and LLDAS are associated with reduced organ damage accrual and improved outcomes in patients (pts).5,6 We report achievement of LLDAS and DORIS in pts with SLE treated with DZP in PHOENYCS GO.
Methods: PHOENYCS GO, a 48-wk, randomized, double-blind, PBO-controlled trial, included pts aged ≥16 years with moderate-to-severe, active SLE characterized by persistently active or frequently flaring/relapsing-remitting disease activity despite stable SOC medication (antimalarials, glucocorticoids and/or immunosuppressants). Pts were randomized 2:1 to intravenous DZP 24 mg/kg+SOC or PBO+SOC every 4 wks. Achievement of LLDAS in ≥50% of visits through Wk 48, achievement of LLDAS by visit, cumulative number of visits in LLDAS through Wk 48, and achievement of DORIS7 by visit are reported.
Results: The study was completed on treatment through Wk 48 by 85.4% (182/213) of pts randomized to DZP+SOC and 79.6% (86/108) randomized to PBO+SOC. Baseline characteristics were comparable between the groups (full analysis set; DZP+SOC: n=208; PBO+SOC: n=107; Table).Through 48 wks, 23.6% of pts receiving DZP+SOC achieved LLDAS in ≥50% of visits vs 15.9% receiving PBO+SOC (nominal p=0.1042). At Wk 48, 40.9% of pts receiving DZP+SOC achieved LLDAS vs 19.6% receiving PBO+SOC (nominal p < 0.0001; Figure 1). As early as Wk 12, greater proportions of pts receiving DZP+SOC achieved LLDAS vs PBO+SOC (nominal p < 0.05). The proportion of DZP+SOC LLDAS-responders continued to rise to Wk 48, while in PBO+SOC pts, achievement of LLDAS plateaued from Wk 28. The least square mean (SE) cumulative number of visits in LLDAS through Wk 48 was greater in pts receiving DZP+SOC (2.9 [0.2]) vs PBO+SOC (1.8 [0.3]; nominal p=0.0016; Figure 1). A higher proportion of pts receiving DZP+SOC vs PBO+SOC achieved DORIS at Wk 48 (19.2% vs 8.4%; nominal p=0.0056), as well as at Wks 20, 32, and 36 (nominal p < 0.05; Figure 2).
Conclusion: Treatment with DZP+SOC resulted in higher rates of achievement and time in the prognostically important endpoints of LLDAS and DORIS vs PBO+SOC. These findings provide further insights into the potential benefits of treatment with DZP for pts with SLE.References: 1. Cutcutache I. Arthritis Rheumatol 2023;75 (suppl 9); 2. Powlesland AS. Annals Rheum Dis 2024;83 (suppl 1):261; 3. Clowse M. Arthritis Rheumatol 2024;76 (suppl 9); 4. Parra Sánchez AR. Rheumatol Ther 2023;10:1459–77; 5. Fanouriakis A. Ann Rheum Dis 2024;83:15–29; 6. Tani C. Lupus Sci Med 2018;5:e000234; 7. van Vollenhoven RF. Lupus Sci Med 2021;8:e000538.
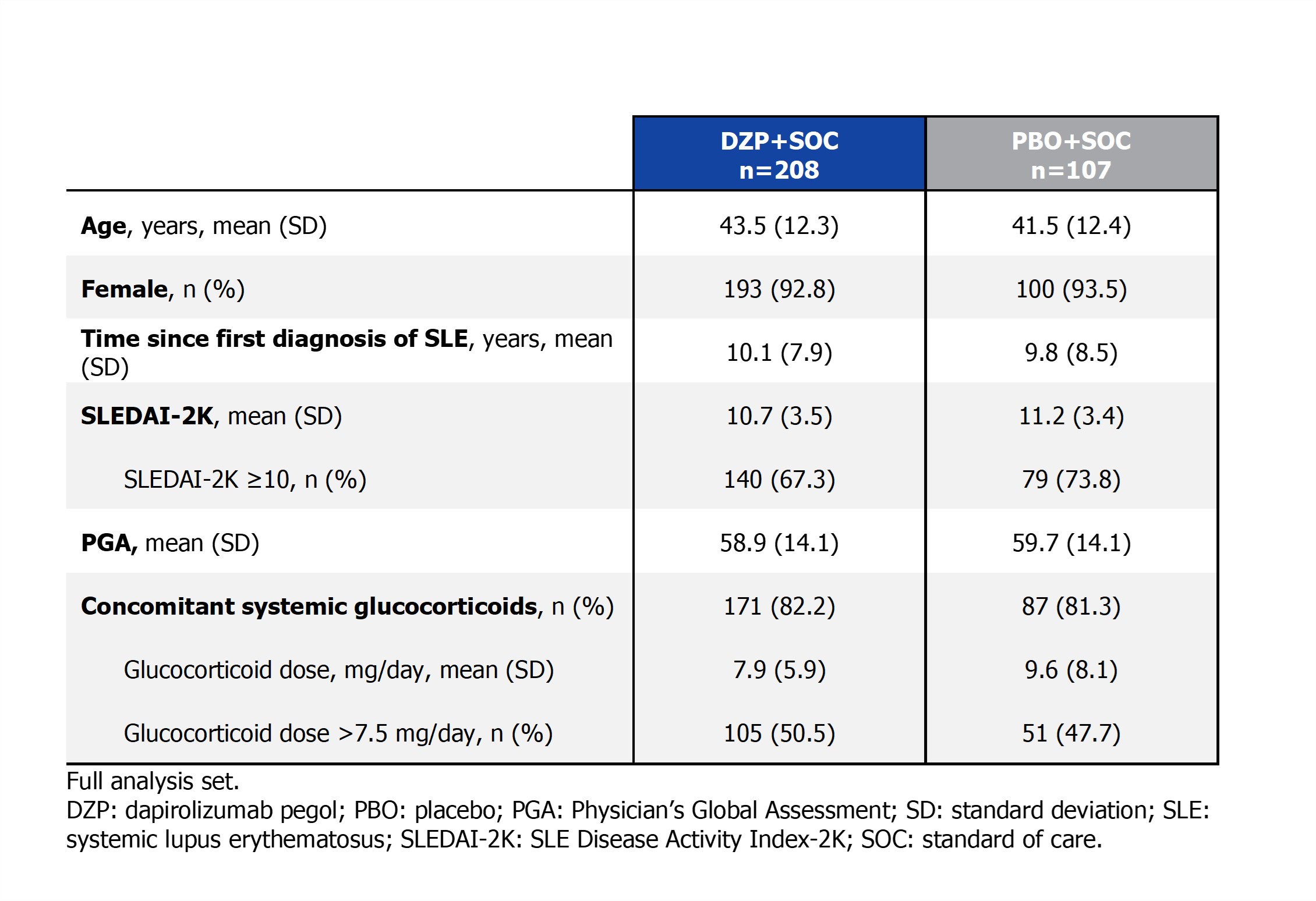 Table. Baseline demographics and disease characteristics
Table. Baseline demographics and disease characteristics
.jpg) Figure 1. (A) Achievement of LLDAS by visit and (B) cumulative number of visits in LLDAS through Week 48
Figure 1. (A) Achievement of LLDAS by visit and (B) cumulative number of visits in LLDAS through Week 48
.jpg) Figure 2. Achievement of DORIS by visit
Figure 2. Achievement of DORIS by visit
To cite this abstract in AMA style:
Morand E, Carter L, Dall'Era M, Petri M, Vital E, Jimenez T, Gaiha-Rohrbach J, Lauwerys B, Nelde A, Stach C, van Vollenhoven R. Achievement of Low Disease Activity and Remission in Patients with Systemic Lupus Erythematosus Treated with Dapirolizumab Pegol: 48-Week Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/achievement-of-low-disease-activity-and-remission-in-patients-with-systemic-lupus-erythematosus-treated-with-dapirolizumab-pegol-48-week-results-from-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-low-disease-activity-and-remission-in-patients-with-systemic-lupus-erythematosus-treated-with-dapirolizumab-pegol-48-week-results-from-a-phase-3-trial/
