Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: PsA is a chronic inflammatory disease with a significant burden on patients’ (pts) health-related quality of life (HRQoL).1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A, has demonstrated sustained efficacy to 2 years in clinical trials of pts with PsA.2–3 BKZ has been approved for treatment of PsA in Europe since June 2023, and the US since September 2024.4,5 SPEAK is the first prospective, observational study in routine clinical practice in Europe evaluating HRQoL and clinical outcomes with BKZ in pts with active PsA or axial spondyloarthritis (axSpA). We report interim data from SPEAK, describing characteristics of pts with PsA at BKZ treatment initiation and HRQoL to 24 weeks (wks) in a real-world setting.
Methods: SPEAK is an ongoing 52-wk, multi-country, observational study in Belgium, Czechia, France, Germany, Greece, Spain, and the United Kingdom. This planned interim analysis reports data to April 2, 2025 (~50% enrolment). Adult pts with active PsA or axSpA who initiated BKZ in routine clinical practice could be included if receiving treatment per label (subcutaneous BKZ 160 mg every 4 wks). We report PsA Impact of Disease 12-Item Questionnaire total score (PsAID-12; 0–10), Short Form 36-item Health Survey Physical Component Summary (SF-36 PCS, norm-based T-scores with mean [SD]: 50 [10] in the US general population), and Pt Global Assessment of Disease Activity score (PGADA; 0–100) for pts with PsA to 24 wks. Other clinical outcomes were collected but not available for the data cut. Baseline (BL) characteristics and adverse events are also reported.
Results: By April 2025, BL characteristics were available for 376 pts with PsA (Table). Overall, 80 were biologic/targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) naïve, 89 had 1 previous b/tsDMARD and 207 had ≥2 previous b/tsDMARDs.Improvements in PsAID-12 total score were observed to 24 wks, with mean (SD) CfB at Wk 24 of −1.9 (2.0). SF-36 PCS scores improved to Wk 24 (mean [SD] CfB: +4.6 [7.9]), as did PGADA scores (Mean [SD] CfB: −17.5 [23.8]); Figure; BL values in Table). TEAEs were reported in 61.4% pts; in 34.8% they were deemed drug-related. One pt reported uveitis (0.3%). 43 pts reported Candida infections (11.4%), including 29 with oral candidiasis (7.7%; 3.5% led to BKZ discontinuation). There were no cases of IBD or suicidal ideation/behavior.
Conclusion: In this first report of HRQoL outcomes in an observational setting of BKZ-treated pts with PsA in routine clinical practice, BKZ treatment led to improvements in pt-reported outcomes at 24 wks, with changes observed as early as Wk 2, demonstrating rapid HRQoL improvements. The real-world findings complement data from phase 3 trials, for both HRQoL and safety.References: 1. Coates LC. CME Rheum 2017;17:65–70; 2. McInnes IB. Lancet 2023;401:25–37; 3. Merola JF. Lancet 2023;401:38–48; 4. EMA. Bimekizumab Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf; 5. FDA. 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761151s009lbl.pdf.
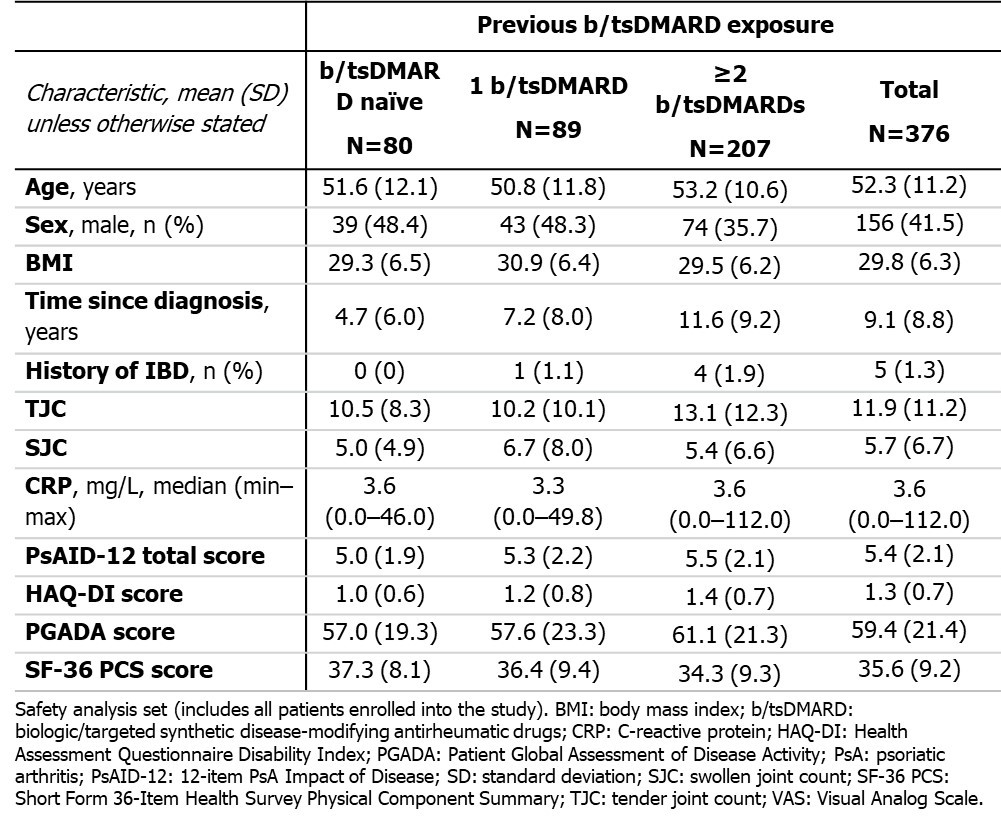 Table. Baseline characteristics of PsA patients in the SPEAK study
Table. Baseline characteristics of PsA patients in the SPEAK study
.jpg) Figure. CfB in PsAID-12, SF-36 PCS and PGADA scores to week 24 (OC)
Figure. CfB in PsAID-12, SF-36 PCS and PGADA scores to week 24 (OC)
To cite this abstract in AMA style:
Baraliakos X, Kiltz U, de Vlam K, Ruyssen-Witrand A, Ramirez Garcia F, Walsh J, Poddubnyy D, Nicholls D, Oortgiesen M, Castagna F, Bonny M, Besson H, Lambert J, Bajracharya R, Öhagen P, Tillett W. An Interim Analysis of Health-Related Quality of Life Outcomes from an International Multicentre Observational Study in Patients with Psoriatic Arthritis Initiating Bimekizumab in Real-World Clinical Practice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/an-interim-analysis-of-health-related-quality-of-life-outcomes-from-an-international-multicentre-observational-study-in-patients-with-psoriatic-arthritis-initiating-bimekizumab-in-real-world-clinical/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-interim-analysis-of-health-related-quality-of-life-outcomes-from-an-international-multicentre-observational-study-in-patients-with-psoriatic-arthritis-initiating-bimekizumab-in-real-world-clinical/
