Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: A treat-to-target (T2T) strategy in psoriatic arthritis (PsA) is supported by International PsA recommendations but not widely practiced in clinic. The aim of the Multicentre Observational Initiative in Treat-to-Target Outcomes in Psoriatic Arthritis (MONITOR-PsA) cohort was to assess clinical and patient-reported outcomes with a pragmatic implementation of a T2T approach.
Methods: Adult patients from 11 UK rheumatology departments were included if they had a clinical diagnosis of active PsA ( ≥ 1 tender or swollen joints or enthesis); not previously had treated with DMARDs for articular disease. The MONITOR-PsA cohort patients were assessed every 12 weeks for one year and were treated with current standard step-up care. Initial treatment consisted of methotrexate, then the addition of or switch to another csDMARD, and then potentially a switch to a bDMARD dependent on disease activity(Figure 1). The primary outcome was the proportion of patients achieving the PsA Disease Activity Score (PASDAS) ‘good’ response at 48 weeks. Key secondary endpoints were the proportion achieving PASDAS moderate response at 48 weeks and PASDAS continuous outcome 48 weeks. Exploratory outcomes included the proportion achieving MDA and DAPSA remission (≤4) at 48 weeks, the number of patients achieving a 50% decrease in 50% in dactylitis/enthesitis scores, the proportion achieving PSAID patient acceptable symptom state (≤4) and the difference between baseline and 48 weeks for radiographic outcomes (score of erosions, joint space narrowing score, modified Sharp-van der Heijde score).
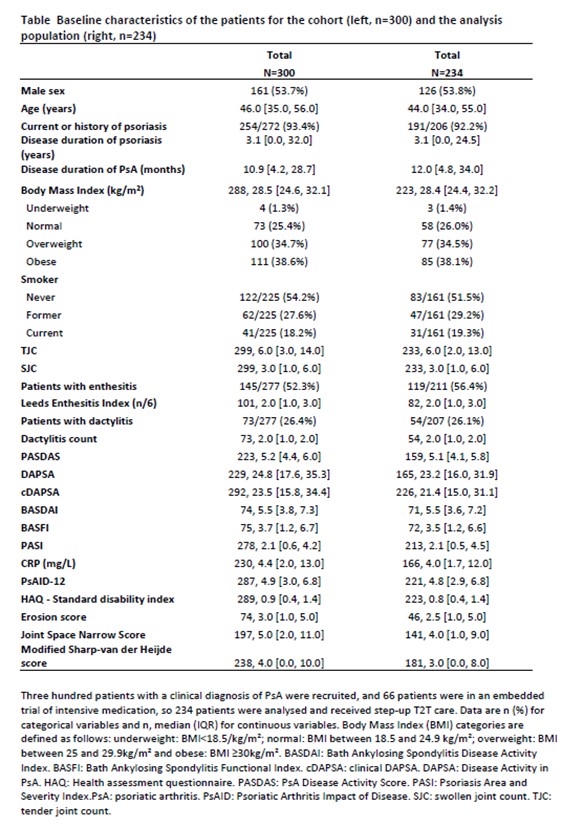
Results: We recruited 300 patients, 197 with polyarticular PsA and 103 with oligoarticular (< 4 active joints) PsA from 18th April 2018 to 23rd August 2022. Sixty-six patients were in an embedded trial of intensive medication, so the analysis population consisted of 234 patients receiving step-up T2T care. At baseline, PsA was moderately active with a median PASDAS of 5.1 [4.1, 5.8] and a median DAPSA of 23.2 [16.0, 31.9], and 19.7% of patients had structural damage (Table). At 48 weeks, 77/234 patients (33.0%) were treated with methotrexate alone and 45/234 (19.0%) were treated with bDMARDs, mainly TNFi (38/45; 84.5%) (Figure 2). The proportion of patients achieving the PASDAS ‘moderate’ and ‘good’ responses at 48 weeks were 49/146 (33.6%) and 54/146 (37.0%), respectively. DAPSA remission and MDA were achieved in 35/107 (32.7%) and 70/147 (47.6%) patients at 48 weeks, respectively. 37/54 (68.5%) and 68/119 (57.1%) of patients achieved a 50% decrease in dactylitis and enthesitis counts, respectively. The majority of patients (117/168 or 69.6%) achieved PSAID patient acceptable symptom state (≤4). No structural progression was noted from baseline to 48 weeks.
Conclusion: In this pragmatic routine implementation of a T2T approach, we report results close to the T2T outcomes in the TICOPA trial (a PASDAS ‘good’ response, 37.0% vs 46% and or a PASDAS ‘moderate’ response 33.6% vs 33%), respectively MONITOR-PsA vs TICOPA), suggesting that a pragmatic T2T approach can be implemented with good outcomes in routine practice.
.jpg) Figure 1 Treat to target strategy
Figure 1 Treat to target strategy
.jpg) Figure 2 – Medication use in the MONITOR cohort
Figure 2 – Medication use in the MONITOR cohort
To cite this abstract in AMA style:
Letarouilly J, Saeedi E, Hurtubise R, James L, Gullick N, Jadon D, Tillett W, Sinomati Y, Tucker L, Mian N, Massa S, Coates L. Real-World Treat-to-Target Strategy in Psoriatic Arthritis: 48-week Results from the MONITOR-PsA Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-treat-to-target-strategy-in-psoriatic-arthritis-48-week-results-from-the-monitor-psa-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treat-to-target-strategy-in-psoriatic-arthritis-48-week-results-from-the-monitor-psa-cohort/