Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Guselkumab (GUS), a fully human IL-23p19-subunit inhibitor, has demonstrated efficacy in significantly improving psoriatic arthritis (PsA) signs and symptoms with two dosing regimens: 100 mg every 4 weeks (Q4W) or 100 mg at Week (W)0, W4, then Q8W, in the pivotal Phase 3 DISCOVER-1&2 studies.1,2 SOLSTICE, an ongoing Phase 3b, multicenter, randomized, double-blinded placebo-controlled study, was designed to evaluate the efficacy and safety of GUS Q4W and Q8W in a dedicated participant (pt) population with active PsA who were inadequate responders (IR [inadequate efficacy and/or intolerance]) to one prior tumor necrosis factor inhibitor (TNFi).
Methods: Adults (≥18 years) with active PsA (≥3 swollen joints; ≥3 tender joints; C-reactive protein [CRP] ≥0.3 mg/dL) who were TNFi-IR to one prior TNFi were eligible to be enrolled from 128 sites across 12 countries. Pts were randomized 1:1:1 to GUS 100 mg Q4W; GUS at W0, W4, then Q8W; or placebo (PBO) with crossover to GUS 100 mg Q4W at W24. Randomization was stratified by baseline use of conventional synthetic disease-modifying antirheumatic drugs. The primary endpoint was defined as achievement of an ACR20 response at W24. Major secondary endpoints included the proportions of pts achieving ACR50/70, Investigator’s Global Assessment of psoriasis response (IGA 0/1 and ≥2-grade improvement), ≥90% improvement in Psoriasis Area and Severity Index (PASI 90), and minimal disease activity (MDA) at W24.
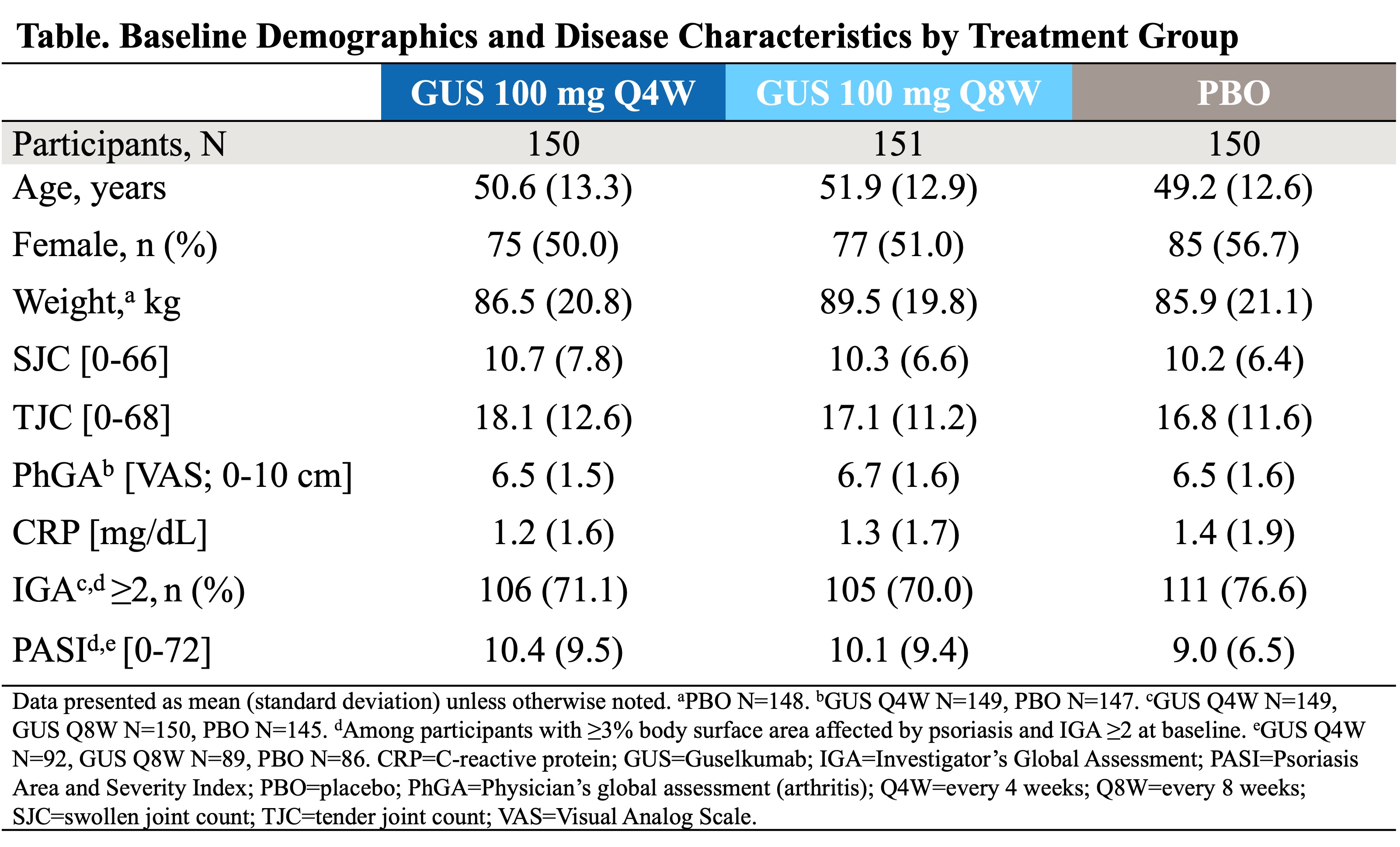
Results: A total of 451 pts (Q4W n=150, Q8W n=151, PBO n=150) were included in the analysis set. Baseline characteristics were well balanced among treatment groups (mean at baseline: 50.6 years, 87.3 kg, 52.6% female; Table). Discontinuation rates were comparable between GUS Q4W (5.3% [8/150]), Q8W (4.6% [7/151]), and slightly higher for PBO (7.4%, [11/149]). Significantly greater proportions of pts in the Q4W and Q8W vs PBO groups achieved ACR20 at W24 (58.6% and 62.2% vs 34.8%, respectively), ACR50 (31.4% and 32.1% vs 12.2%, respectively), and ACR70 (17.5% and 17.3% vs 2.0%, respectively); p< 0.001 for all (Figure 1a). Through W24, Q4W and Q8W groups start achieving ACR20 as early as W4, compared to the PBO group (Figure 1b). GUS-treated pts in the Q4W and Q8W treatment groups also had significantly greater response rates at W24 vs PBO for MDA (18.8% and 23.9% vs 5.4%, respectively), IGA 0/1 response (50.0% and 57.3% vs 17.4%, respectively), and PASI 90 (49.4% and 45.5% vs 12.0%, respectively); p< 0.001 for all (Figure 2). Through W24, 49.5% of pts had ≥1 adverse event (AE), and 2.6% had ≥1 serious adverse event (SAE). Overall, the frequency of AEs and SAEs was similar across treatment groups and comparable to PBO.
Conclusion: In the TNFi-IR PsA population from SOLSTICE, GUS demonstrated superior efficacy vs PBO for improving signs and symptoms of peripheral arthritis and skin psoriasis. Both the GUS Q4W and Q8W dosing regimens were equally efficacious in this treatment refractory PsA population. Safety findings were consistent with the known safety profile of GUS in pts with psoriatic disease.3References: 1. Deodhar A, et al. Lancet. 2020;1115-25. 2. Mease PJ, et al. Lancet. 2020;395:1126–36. 3. Strober, B, et al. Drug Safety. 2024;47, 39–57.
To cite this abstract in AMA style:
Ogdie A, Merola J, Mease P, Ritchlin C, Scher J, Parnell Lafferty K, Chan D, Chakravarty S, Langholff W, Wang Y, Choi, MD, PhD, FAAD O, Krol Y, Gottlieb A. Efficacy and Safety of Guselkumab in Patients with Active Psoriatic Arthritis and Inadequate Response and/or Intolerance to One Prior Tumor Necrosis Factor Inhibitor [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-guselkumab-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-and-or-intolerance-to-one-prior-tumor-necrosis-factor-inhibitor/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-guselkumab-in-patients-with-active-psoriatic-arthritis-and-inadequate-response-and-or-intolerance-to-one-prior-tumor-necrosis-factor-inhibitor/

.jpg)
.jpg)