Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Available evidence hints towards the positive results of the use of sulfasalazine in the management of patients with psoriatic arthritis (PsA). We did a systematic review and meta-analysis to determine the possible safety and effectiveness of sulfasalazine for the treatment of PsA.
Methods: We conducted a thorough literature search using PubMed and Cochrane Library to retrieve and review all the articles through July 2024. Each of the studies in our review was to report different measures to determine the safety and efficacy of sulfasalazine by using 95% CIs. PRISMA flow guidelines were followed and used to assess the methodological quality of evidence and reporting Quality. The GRADE system was used to appraise the certainty of evidence.
Results: Our literature search produced 17 results and a final of 3 studies were included in our analysis. All the studies were prospective RCTs with a total population of 380 patients with male dominant population. There was a significant and persistent treatment effect on ESR, as evidenced by the overall standardized mean difference (SMD) of -0.47 (95% CI: [-0.68, -0.26], Z = 4.41, P < 0.0001) and low heterogeneity (Chi² = 1.88, P = 0.39, I² = 0%). The overall pooled mean difference was found to be -1.09 (95% CI: -2.95 to 0.77) and a p-value of 0.25 in case of Ritchie Index, showing no statistically significant benefit of Sulfasalazine over placebo. Based on the three studies included, overall effect size was found to be -0.13 (95% CI: -0.34 to 0.08), showing no notable variation among the groups. Heterogeneity was low (I² = 0%, p = 0.93), and thus result was not statistically important (Z = 1.23, p = 0.22). Two of the studied showed a low risk of bias where as one showed a high risk.
Conclusion: Sulfasalazine has some benefits in reducing systemic inflammation in people with psoriatic arthritis (PsA), as shown by an improved erythrocyte sedimentation rate (ESR). However, its efficacy as a stand-alone treatment is limited due to its negligible effect on important clinical symptoms such as joint pain, stiffness, and soreness. For mild to moderate PsA, sulfasalazine may be helpful as adjuvant therapy, but in more severe instances, it is not enough for complete management. To elucidate its function in PsA management, more studies involving bigger and more varied populations are required.
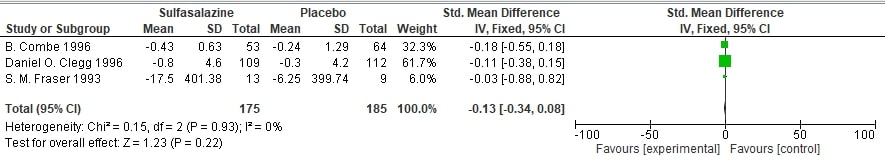 Forest Plot of effect on Morning Stiffness
Forest Plot of effect on Morning Stiffness
.jpg) Forest Plot of effect on Ritchie Articular Index
Forest Plot of effect on Ritchie Articular Index
To cite this abstract in AMA style:
Qasim R, Mohib K, Naqvi U, Anjum A, Fatima M. Evaluating the Efficacy of Sulfasalazine Compared to Placebo in the Treatment of Psoriatic Arthritis in Adults: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluating-the-efficacy-of-sulfasalazine-compared-to-placebo-in-the-treatment-of-psoriatic-arthritis-in-adults-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluating-the-efficacy-of-sulfasalazine-compared-to-placebo-in-the-treatment-of-psoriatic-arthritis-in-adults-a-systematic-review-and-meta-analysis/

.jpg)