Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Treatment I (0801–0806)
Session Type: Abstract Session
Session Time: 2:15PM-2:30PM
Background/Purpose: Anifrolumab and belimumab are biologic agents approved for systemic lupus erythematosus (SLE), yet their comparative safety profiles, particularly regarding infection risks, remain inadequately characterized in real-world settings.
Methods: We conducted a retrospective cohort study using data from the TriNetX research network. Patients diagnosed with SLE between January 1, 2000, and June 30, 2024, with at least two diagnoses were included. We identified new users of anifrolumab or belimumab between August 1, 2021, and June 30, 2024, excluding patients with infection before the index date, patients hospitalized within 6 months before the index date, and patients who died before the index date. Propensity score matching was employed to control for confounding factors. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI), and Kaplan-Meier analysis was used to assess cumulative infection probabilities. The primary outcome was infection occurrence (including herpes zoster, influenza, pneumonia, tuberculosis, sepsis, urinary tract infection, and COVID-19), while secondary outcomes included mortality and hospital inpatient services utilization.
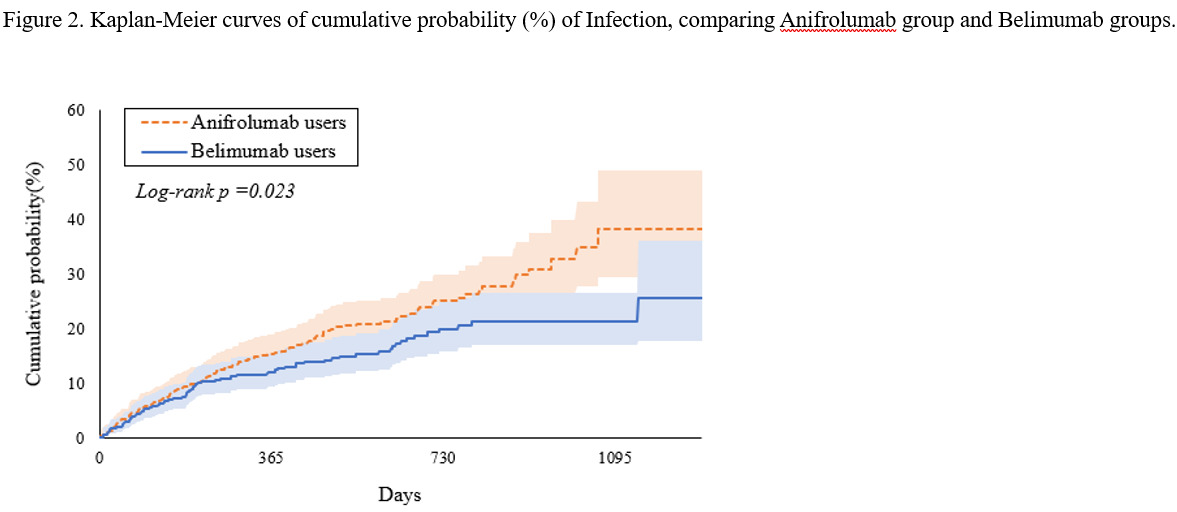
Results: After applying exclusion criteria and propensity score matching, the final analytic cohort included 481 patients in each treatment group. After matching, anifrolumab users showed a significantly higher risk of infection compared to belimumab users (HR 1.40, 95% CI: 1.05-1.88), with 3-year cumulative infection rates of 38.3% and 21.3%, respectively. Analysis of specific infections revealed significantly increased risks of herpes zoster (HR 3.944, 95% CI: 1.111-13.996) and COVID-19 (HR 1.663, 95% CI: 1.042-2.653) associated with anifrolumab use. Subgroup analysis showed significantly higher infection risk in White patients receiving anifrolumab (HR 1.64, 95% CI: 1.09-2.48). Time-dependent analysis suggested that certain infection risks, such as herpes zoster, may develop or intensify with longer treatment duration. No significant differences in mortality or hospital inpatient services utilization were observed between the treatment groups.
Conclusion: Anifrolumab use in SLE is associated with a higher risk of specific infections, particularly herpes zoster and COVID-19, compared to belimumab, especially with prolonged exposure. These findings underscore the need for vigilant infection monitoring, region-specific preventive strategies, and further research to optimize biologic therapy safety across diverse populations.
To cite this abstract in AMA style:
Hsu T, Huo A, Liao P, Leong P, Wei J. Comparative Risk of Infection-Related Complications in Systemic Lupus Erythematosus Patients Treated with Anifrolumab versus Belimumab: A Target Trial Emulation [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparative-risk-of-infection-related-complications-in-systemic-lupus-erythematosus-patients-treated-with-anifrolumab-versus-belimumab-a-target-trial-emulation/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-risk-of-infection-related-complications-in-systemic-lupus-erythematosus-patients-treated-with-anifrolumab-versus-belimumab-a-target-trial-emulation/

.jpg)
.jpg)