Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Lupus Erythematosus – Treatment I (0801–0806)
Session Type: Abstract Session
Session Time: 1:30PM-1:45PM
Background/Purpose: Chronic GC and IS use increases damage accrual and mortality. The 2023 EULAR SLE recommendations support initiation of biologics such as BEL, a human IgG1λ monoclonal antibody that selectively binds to soluble B-lymphocyte stimulator (BLyS) with proven efficacy in SLE, without prior IS failure in AM- and GC-non-responders. Real-world evidence has shown benefits of initiating BEL without prior IS treatment. This study assessed the efficacy and safety of initiating BEL relative to being on IS at baseline of clinical trials (combined with AM and GC) in patients with SLE.
Methods: This post hoc summary pooled Week 52 data from five Phase 3 trials (BLISS-52: NCT00424476; BLISS-76: NCT00410384; North East Asia: NCT01345253; BLISS-SC: NCT01484496; EMBRACE: NCT01632241) with similar study designs and eligibility criteria, where adults with active SLE were randomized to receive BEL or placebo (PBO) in addition to AM and GC, with or without IS. This summary grouped patients by baseline treatment into an IS cohort (PBO and IS, defined as pre-treatment) and a BEL cohort (BEL 10 mg/kg intravenous or 200 mg subcutaneous and no IS); all patients received AM and GC. Efficacy endpoints were: proportion of patients with SLE Responder Index-4 (SRI-4) response through 52 weeks, time to first SELENA-SLEDAI Flare Index (SFI) flare over 52 weeks, and cumulative and mean GC dose change between baseline and Week 52. AEs and SAEs, inclusive of Phase 2 (NCT00071487) data, and baseline demographic and outcome data were summarized descriptively for the BEL and IS cohorts.
Results: Baseline demographics and clinical characteristics were similar between BEL (Nf552) and IS cohorts (Nf358) (Table 1). Patients had mean (SD) SDI of 0.3 (0.73) and 0.5 (1.03), and median (IQR) duration of SLE of 3.9 [1.2–7.4] and 4.2 [1.6–8.8] years in the BEL and IS cohorts. More patients were SRI-4 responders in the BEL vs IS cohort at most time points (Week 52 difference: 22%) (Figure 1A). In the BEL cohort, numerically fewer patients experienced any SFI flare (BEL: 59% vs IS: 81%), and had longer median [IQR] time to first on-study flare (any severity) (BEL: 85.0 [44.0–168.0] vs IS: 69.0 [32.0–134.0] days). The BEL cohort had a numerically lower cumulative baseline GC dose (× 365 days) vs IS cohort (mean [SD]: 4729 [3268.2] vs 5340 [3613.8] mg), and cumulative dose through Week 52 (mean [SD]: 4536 [3092.1] vs 5679 [5361.2] mg); mean (SD) cumulative GC change from baseline: −192 (2510.8) mg (BEL cohort), and 339 (4725.5) mg (IS cohort) (Figure 1B).Fewer patients experienced AEs and SAEs in the BEL (Nf588) versus IS (Nf395) cohort (AEs: 79% vs 90%; SAEs: 11% vs 20%), with one death reported in the BEL cohort (due to pneumonia; unrelated to treatment). Most common AEs by system organ class (BEL vs IS) were infections and infestations (53% vs 64%), gastrointestinal disorders (23% vs 31%), and musculoskeletal and connective tissue disorders (21% vs 35%).
Conclusion: In line with 2023 SLE EULAR recommendations for earlier biologic use in the treatment paradigm, this post hoc summary adds clinical data supporting the benefits of initiating BEL in patients not receiving IS, in those not well controlled with AM and GC.Funding: GSKOriginal presentation: EULAR 2025
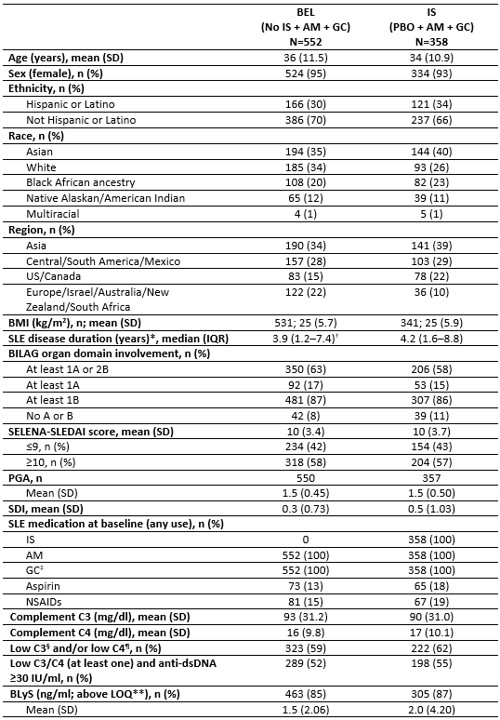 Table 1. Baseline demographics and clinical characteristics.
Table 1. Baseline demographics and clinical characteristics.
*Duration defined as: (screening date/treatment start date − SLE diagnosis date + 1)/365.25; †no data for one patient; ‡comprising intravenous, intramuscular, subcutaneous and oral GC; §defined as < 90 mg/dl; ¶defined as < 10 mg/dl for BLISS-SC, BLISS-North East Asia and EMBRACE, and < 16 mg/dl for BLISS-52 and BLISS-76; **defined as 0.61 ng/ml for BLISS-SC and North East Asia, 0.02048 ng/ml for EMBRACE, and 0.50 ng/ml for BLISS-52 and BLISS-76.
BILAG, British Isles Lupus Assessment Group; BMI, body mass index; C3/C4, complement component C3 and C4; dsDNA, double-stranded DNA; LOQ, limit of quantification; NSAIDs, nonsteroidal anti-inflammatory drugs; PGA, physician global assessment; SDI, The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) damage index; SELENA, Safety of Estrogens in Lupus Erythematosus National Assessment; SLEDAI, SLE Disease Activity Index.
.jpg) Figure 1. SRI-4* responders through Week 52 (A) and cumulative mean change in GC dose from baseline to Week 52 (B).
Figure 1. SRI-4* responders through Week 52 (A) and cumulative mean change in GC dose from baseline to Week 52 (B).
*SRI-4 response was defined as a ≥4-point reduction in SELENA-SLEDAI score from baseline, no new BILAG A organ domain score or 2 new BILAG B scores compared with baseline, and no worsening (increase < 0.3) in PGA score versus baseline.
To cite this abstract in AMA style:
Gatto M, Costenbader K, Schwarting A, Harris J, O'Shea C, Levy R, Doria A. Improved Efficacy and Safety Outcomes in Patients with SLE Treated with Belimumab (BEL) Versus Immunosuppressants (IS), in Addition to Antimalarials (AM) and Glucocorticoids (GC): A Post Hoc Summary of Five Phase 3 Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/improved-efficacy-and-safety-outcomes-in-patients-with-sle-treated-with-belimumab-bel-versus-immunosuppressants-is-in-addition-to-antimalarials-am-and-glucocorticoids-gc-a-post-hoc-summary-o/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improved-efficacy-and-safety-outcomes-in-patients-with-sle-treated-with-belimumab-bel-versus-immunosuppressants-is-in-addition-to-antimalarials-am-and-glucocorticoids-gc-a-post-hoc-summary-o/
