Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes (0789–0794)
Session Type: Abstract Session
Session Time: 1:15PM-1:30PM
Background/Purpose: Psoriasis is a chronic inflammatory skin disease associated with increased risk of metabolic complications, such as diabetes mellitus. However, the impact of specific biologic DMARD therapies on these metabolic outcomes remains poorly understood. Importantly, understanding how psoriatic treatments affect metabolic outcomes may guide therapeutic choices; thus, this study evaluates the risks of diabetes and kidney disease across biologic DMARD therapies.
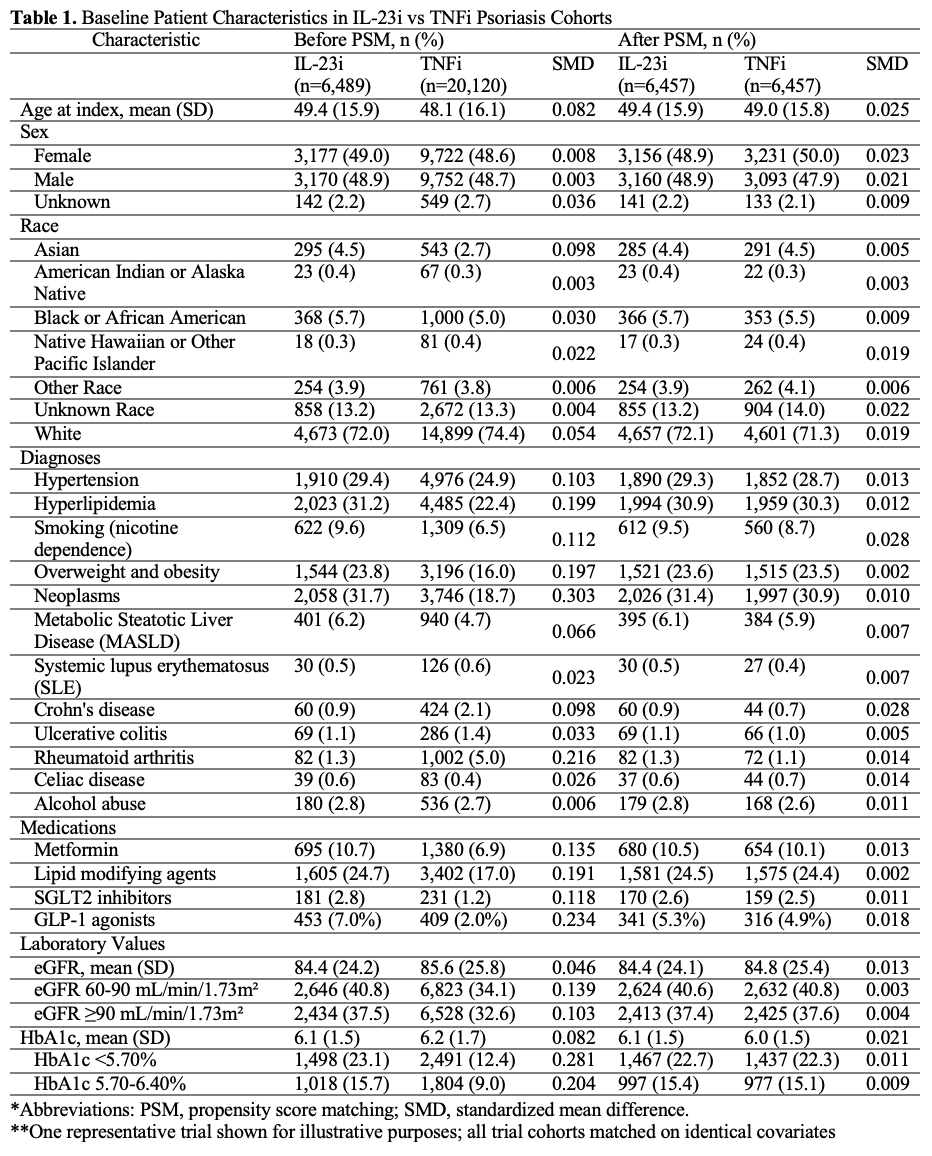
Methods: Utilizing data from 128 healthcare organizations in the TriNetX network (2016-2024), we conducted an emulated trial to evaluate diabetes-related disease incidence among psoriasis patients (n=20,120) treated with interleukin-23 (IL-23i), interleukin-17A (IL-17Ai), or tumor necrosis factor-alpha (TNFi) inhibitors. To emulate randomization, we applied 1:1 propensity score matching using a greedy nearest neighbor algorithm to balance cohorts on demographics, comorbidities, laboratory data, and medication use (Table 1). Primary outcomes included new-onset type 2 diabetes, diabetic nephropathy, chronic kidney disease (CKD), and stage 3–5 CKD. Varicose vein and head laceration diagnoses served as negative control outcomes. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards models.
Results: In an emulated trial of IL-23i versus TNFi treatment (n=6,457 matched pairs), IL-23i treatment was associated with lower risks of diabetes (HR 0.91; 95% CI 0.75–0.99), diabetic nephropathy (HR 0.71; 95% CI 0.48–0.98), and stage 3–5 CKD (HR 0.75; 95% CI 0.58–0.98) (Table 2). In the TNFi versus IL-17Ai emulated trial (n=6,425 pairs), TNFi-treated patients had a higher risk of diabetes (HR 1.16; 95% CI 1.01–1.22) and associated kidney disease outcomes. No significant differences were observed between IL-23i and IL-17Ai treatment. Compared to systemic therapy naïve (STN) patients (n=6,489 pairs), IL-23i treatment was associated with reduced risks of diabetes (HR 0.89; 95% CI 0.80–0.98), diabetic nephropathy (HR 0.78; 95% CI 0.60–0.97), and stage 3–5 CKD (HR 0.76; 95% CI 0.62–0.92) (Table 3); IL-17Ai treatment showed similarly protective effects for diabetes (HR 0.90; 95% CI 0.83–0.98), diabetic nephropathy (HR 0.74; 95% CI 0.57–0.96), and stage 3–5 CKD (HR 0.88; 95% CI 0.75–0.99) risk (n=6,402 pairs). TNFi treatment showed no significant differences compared to STN patients. No differences were observed in negative control outcomes across comparisons.
Conclusion: These emulated target trials suggest that IL-17Ai and IL-23i therapies are associated with lower risks of incident type 2 diabetes and kidney disease compared to TNFi or systemic therapy naïve approaches. These findings may support the consideration of metabolic outcomes in biologic DMARD treatment selection for psoriasis patients.
To cite this abstract in AMA style:
Mahajan A, Bates D, Nambudiri V, LaChance A, Sparks J, Piette E. Diabetes Risk in Psoriasis Patients Treated with Biologic Disease-Modifying Antirheumatic Drugs: Target Trial Emulation Using Nationwide Data [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/diabetes-risk-in-psoriasis-patients-treated-with-biologic-disease-modifying-antirheumatic-drugs-target-trial-emulation-using-nationwide-data/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diabetes-risk-in-psoriasis-patients-treated-with-biologic-disease-modifying-antirheumatic-drugs-target-trial-emulation-using-nationwide-data/

.jpg)
.jpg)