Session Information
Date: Sunday, October 26, 2025
Title: (0731–0764) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with giant cell arteritis (GCA) can relapse despite glucocorticoids, methotrexate and tocilizumab treatment. The JAK/STAT signalling pathway is involved in the pathogenesis of GCA, and JAK inhibitors (JAKi) are a potential treatment alternative. Our Objective was to evaluate the effectiveness and safety of JAKi in GCA.
Methods: Real-world, retrospective clinical practice study of patients with GCA treated with JAKi. Outcomes assessed included clinical remission and EULAR remission (clinical remission+CRP and ESR normalization), disease relapse and safety. A literature search for other JAKi-treated GCA cases was conducted in PubMed from inception to 11/30/2024.
Results: We include 40 patients (35 females/87.5%), mean age, 72.2 years, relapsing disease 40 (100%) that received JAKi. The initial JAKi was baricitinib (n=15), tofacitinib (n=10) and upadacitinib (n=15) (Table and Figure). After a mean±SD follow-up of 12.4±6.8 months, 24 (60%) achieved a maintained remission, and 16 (40%) patients discontinued the initial JAKi due to relapse (n=12, 30%) or severe adverse events (SAEs) (n=4, 10%) including elevation of liver enzymes (n=1), disseminated herpes zoster (n=1), glioblastoma multiforme (n=1), and palpitations/dyspnea (n=1). Two patients developed an urinary tract infection and one patient anemia without requiring permanent JAKi discontinuation. The 12 patients failing the initial JAKi were switched to an alternative JAKi (n=2), biologic therapy (n=8) and azathioprine (n=1). One patient discontinued JAKi therapy due to sustained remission.The literature review identified another 37 GCA patients (26 females, mean age 71.4 years) treated with JAKi, mostly with baricitinib (n=35). Most of these patients benefited from JAKi therapy (Table). With respect to relevant adverse events, one patient suffered an Aspergillus fumigatus infection, other patient an Enterococcus faecalis bacteremia, and other thrombocytopenia (Table).
Conclusion: This real-world analysis suggests that JAKi could be effective and relatively safe in GCA, including patients failing to other immunosuppressive therapies.References1. Calderón-Goercke M, et al. Clin Exp Rheumatol. 2023 Apr;41(4):829-836. doi: 10.55563/clinexprheumatol/oqs8u92. Loricera J et al. Clin Exp Rheumatol. 2014 May-Jun;32(3 Suppl 82):S79-89. Epub 2014 May 15. PMID: 24854377.3. Prieto Peña D, et al. Clin Exp Rheumatol. 2021 Mar-Apr;39 Suppl 129(2):69-754. Loricera J, et al. Ther Adv Musculoskelet Dis. 2022 Jul 22;14:1759720X221113747. doi: 10.1177/1759720X221113747
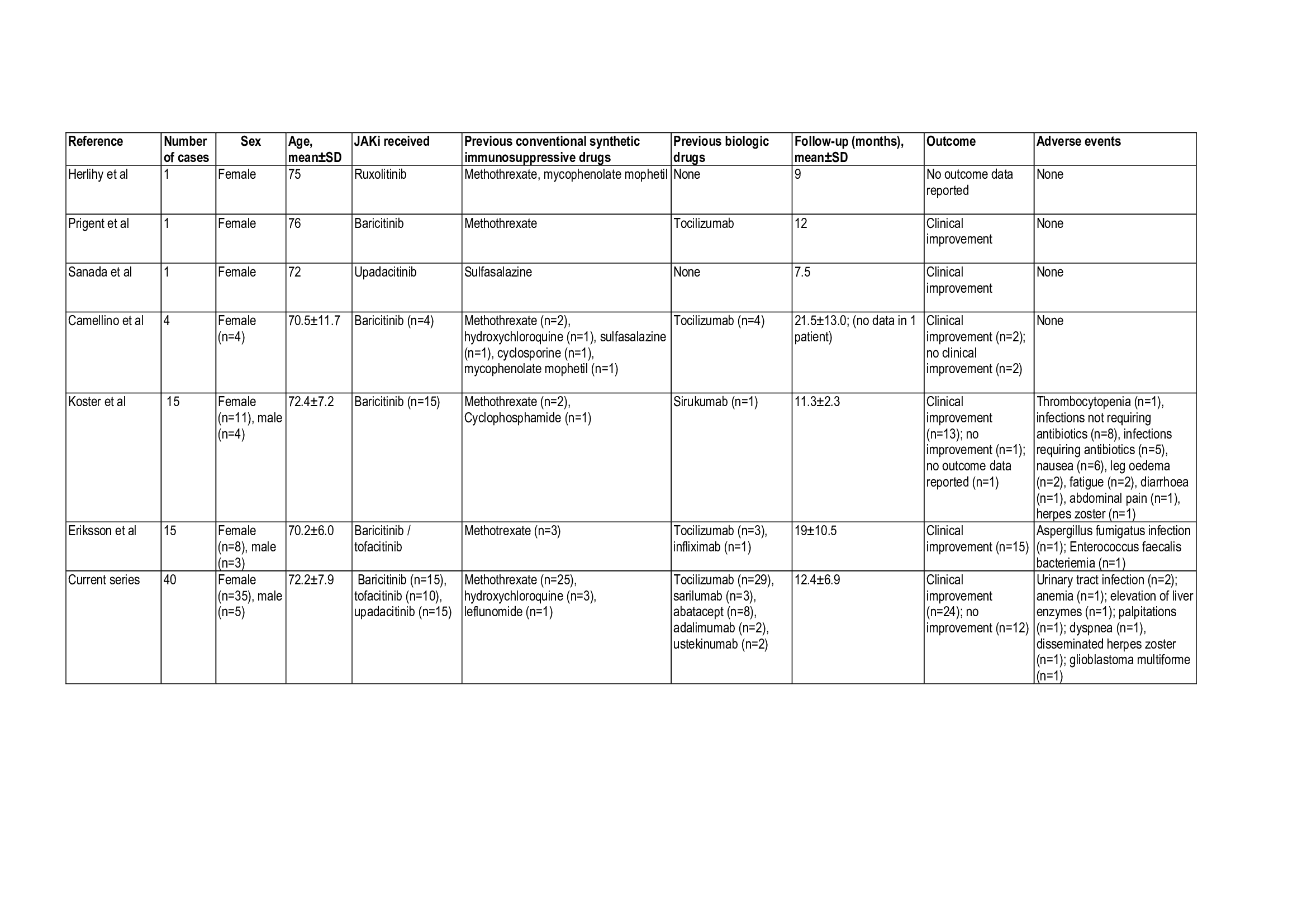 TABLE. Current series and literature review of patients with GCA treated with JAKi.
TABLE. Current series and literature review of patients with GCA treated with JAKi.
.jpg) FIGURE. Flow chart of the 40 GCA patients treated with JAKi.
FIGURE. Flow chart of the 40 GCA patients treated with JAKi.
To cite this abstract in AMA style:
Serrano-Combarro A, Lopez Gutierrez F, Loricera J, Tofade t, Prieto-Peña D, Romero-Yuste S, de Miguel E, Riveros frutos A, FREITES D, Ferraz Amaro I, Castañeda S, Labrador-Sanchez E, Maiz O, Becerra-Fernández E, Narváez J, Galíndez Agirregoikoa E, González I, Urruticoechea-Arana A, Ramos Calvo A, Moya Albarado P, Unizony S, Blanco R. Effectiveness and Safety of Janus Kinase Inhibitors in Giant Cell Arteritis. Real-World Clinical Practice Study and Literature Review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-janus-kinase-inhibitors-in-giant-cell-arteritis-real-world-clinical-practice-study-and-literature-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-janus-kinase-inhibitors-in-giant-cell-arteritis-real-world-clinical-practice-study-and-literature-review/
