Session Information
Date: Sunday, October 26, 2025
Title: (0357–0386) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The purpose of Research Accelerated by You (RAY) is to amplify awareness about clinical research and trials and ensure lupus patient partnership in therapeutic development. However, Black/ African Americans (B/AA) make up only 16% of RAY participants, a notable disparity as B/AAs account for 30-43% of the lupus population. To address the lack of representativeness in race/ethnicity amongst B/AA in RAY, we explored B/AA motivations and communications preferences driving registry enrollment decision-making.
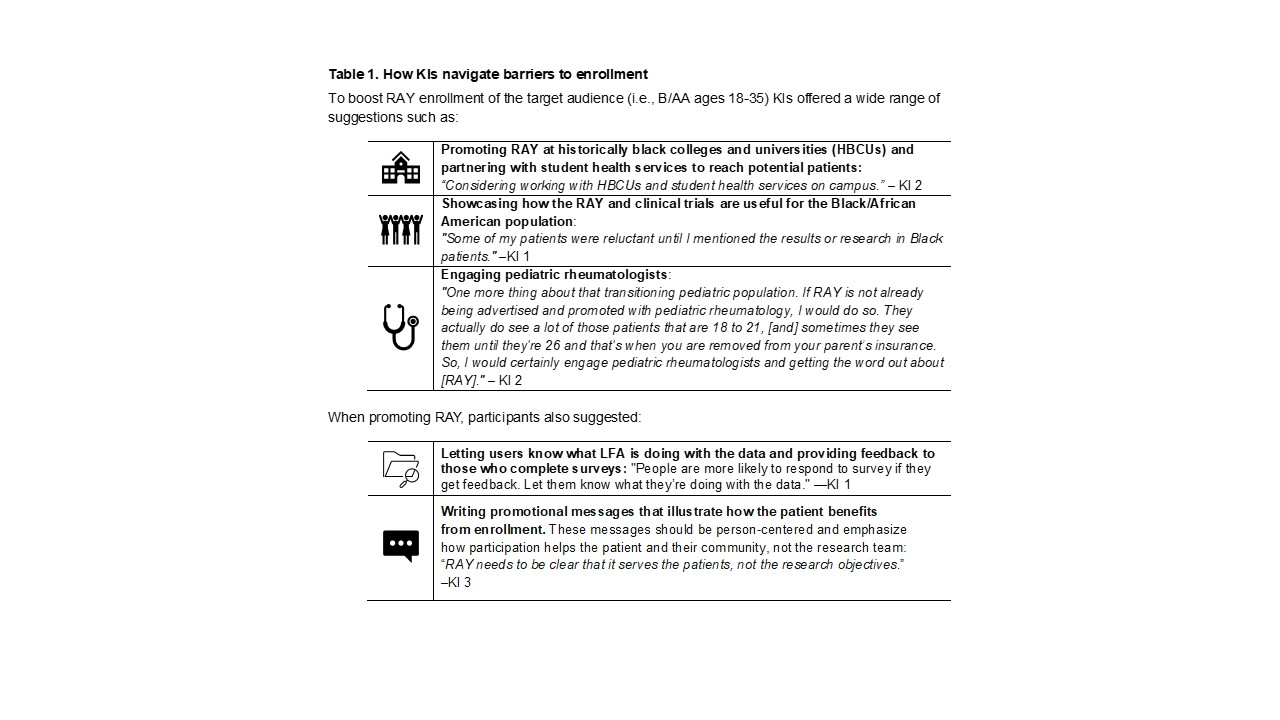
Methods: We conducted two focus groups with people living with lupus (PWL) who were already enrolled in RAY or a part of the Lupus Research Action Network (LRAN), a patient peer-to-peer network, but not enrolled in RAY. All focus group (FG) participants were B/AA females aged 25-46 years (n=4 each). Key informant (KI) interviews were also conducted with PWL with no affiliation with the Lupus Foundation of America (LFA)(n=1) and registry directors at academic medical centers (n=3). Audio-recordings were transcribed and cleaned using Otter.ai. Manual content analysis followed an inductive approach, allowing themes to emerge from data rather than predetermined categories for FGs and KIs. Resulting themes with exemplary quotes are presented in Tables 1-4.
Results: Three overarching themes emerged:1.Communication Preferences: Participants receive most of their lupus information from the LFA’s emails, social media, and website, in addition to email/text alerts from other research institutions. Furthermore, some participants also noted that their family members/friends have sent them lupus materials; however, most shared they prefer to receive lupus-related information from their healthcare team. 2.Building Trust and Rapport: KIs mentioned this theme as essential to encouraging registry and clinical trial enrollment. To build rapport, KIs discussed the importance of speaking with patients face-to-face about enrollment, educating potential participants about patient protections, and explaining the history of mistrust as it relates to the medical profession and the B/AA community in the U.S. 3.Curating Messages/Imagery that Resonate: After reviewing RAY marketing materials, participants stated models who look indisputably like the target audience (i.e., B/AA PWL) are preferred. Additionally, some participants mentioned they prefer the models to feature lupus symptoms such as having a “rash” or “swelling around the eyes.” Participants preferred messaging demonstrating how RAY benefited the patient rather than the researcher, and they favored clear and straightforward content. To improve messaging, participants suggested avoiding the notion that lupus is “easy” and adding language centering the target population.
Conclusion: B/AA PWL may be more inclined to enroll in registries and clinical trials if they receive clear and transparent information from their healthcare team about the benefits and risks of participation, particularly as they relate to B/AA PWL. Centering the experience of B/AA PWL in registry and clinical communication materials is a key theme of importance to potential study participants and may improve enrollment in the registry.
To cite this abstract in AMA style:
Agyemang S, Miller M, Justin T, Oberholtzer L, Buie J. Improving Participation in The Lupus Foundation of America’s Research Accelerated by You (RAY) Patient Registry By Understanding Patient Preference in Communication Strategies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/improving-participation-in-the-lupus-foundation-of-americas-research-accelerated-by-you-ray-patient-registry-by-understanding-patient-preference-in-communication-strategies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-participation-in-the-lupus-foundation-of-americas-research-accelerated-by-you-ray-patient-registry-by-understanding-patient-preference-in-communication-strategies/

.jpg)
.jpg)