Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Interleukin-17 (IL-17) and interleukin-23 (IL-23) inhibitors have revolutionized treatment for psoriatic arthritis, axial spondyloarthritis, and moderate-to-severe plaque psoriasis. IL-17 inhibitors block the pro-inflammatory cytokine IL-17, while IL-23 inhibitors target the Th17 pathway via IL-23. Secukinumab (Cosentyx), an IL-17 inhibitor, was FDA-approved in 2015, and Guselkumab (Tremfya), an IL-23 inhibitor, received approval in 2017. Both classes are highly effective, but their distinct mechanisms lead to different safety profiles, guiding treatment choices—especially in patients with comorbidities.
Methods: The FAERS system gathers information on adverse events and medication errors. We conducted a pharmacovigilance study using the FAERS database to evaluate adverse events (AEs) associated with Secukinumab and Guselkumab. Post-marketing reports from 2015 to 2024 were analyzed using disproportionality analysis. Reporting odds ratios (RORs) were calculated to assess drug-event associations. An ROR >1 indicates a stronger association of the drug with the adverse event compared to the other. An ROR < 1 indicates a weaker association of the drug with the adverse event.
Results: As of December 2024, there were a total of 143,401 and 23,064 reported adverse events (AEs) for Secukinumab and Guselkumab, respectively. Secukinumab was associated with a significantly higher ROR of several adverse events compared to Guselkumab, including liver injury (ROR 13.01, 95% CI 6.41–26.42), pericarditis (ROR 12.78, 95% CI 6.41–25.47), hypercholesterolemia (ROR 9.31, 95% CI 5.00–17.35), type 2 diabetes mellitus (ROR 8.49, 95% CI 4.97–14.48), gastrointestinal disorder (ROR 6.26, 95% CI 4.79–8.18), hypertension (ROR 5.42, 95% CI 4.34–6.78), sinusitis (ROR 4.85, 95% CI 4.02–5.85), depression (ROR 2.99, 95% CI 2.34–3.83), asthma (ROR 2.24, 95% CI 1.55–3.24), and lower respiratory tract infection (ROR 2.11, 95% CI 1.83–2.43). Notably, Guselkumab showed higher odds of cerebrovascular accident (ROR 0.68, 95% CI 0.55–0.85) and myocardial infarction (ROR 0.45, 95% CI 0.36–0.57), indicating these were more frequently reported with Guselkumab. There was no statistically significant difference in the risk of pneumonia (ROR 0.97, 95% CI 0.89–1.06) or death (ROR 1.14, 95% CI 0.99–1.31) between the two agents.
Conclusion: This FAERS-based analysis highlights the distinct adverse event profiles of Secukinumab and Guselkumab. Secukinumab showed higher RORs for liver injury, pericarditis, hypercholesterolemia, type 2 diabetes, GI disorders, hypertension, sinusitis, and depression. Guselkumab was more strongly associated with cerebrovascular accidents and myocardial infarctions. No significant differences were found for pneumonia or mortality. These findings underscore the need for individualized risk assessment when choosing between IL-17 and IL-23 inhibitors and highlight the importance of further research into their long-term safety.
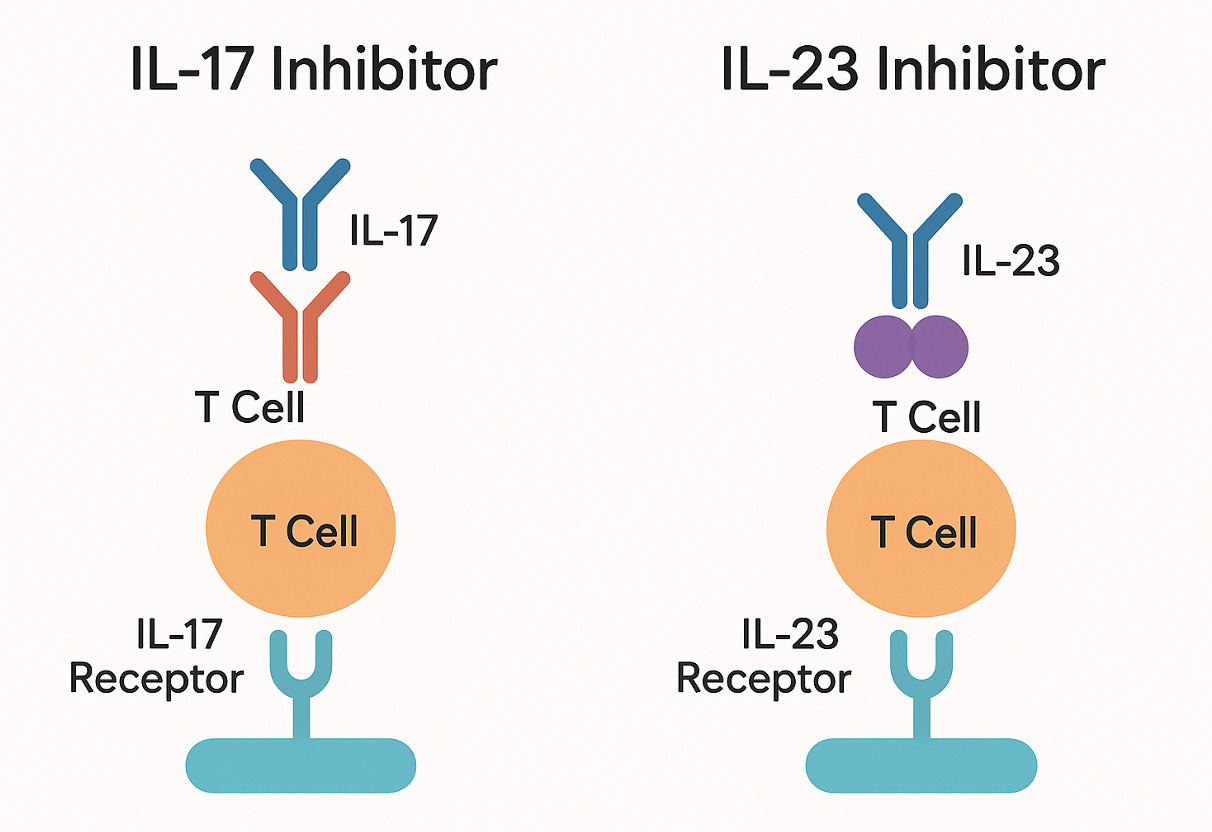 Interleukin-17 and interleukin-23 inhibitors mechanism of action
Interleukin-17 and interleukin-23 inhibitors mechanism of action
.jpg) ROR Results of Secukinumab and Guselkumab
ROR Results of Secukinumab and Guselkumab
To cite this abstract in AMA style:
BARLAS N, Barlas S, Adalier E. Pharmacosurvellience study of FDA Adverse Event Reporting System (FAERS) events of Secukinumab and Guselkumab [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/pharmacosurvellience-study-of-fda-adverse-event-reporting-system-faers-events-of-secukinumab-and-guselkumab/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacosurvellience-study-of-fda-adverse-event-reporting-system-faers-events-of-secukinumab-and-guselkumab/
