Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The home-based therapeutics service (HTS) by a community nurse is part of an ongoing larger initiative—Community Therapeutics Programme (CTP)—spearheaded by the Rheumatology and Immunology Department in Singapore General Hospital. CTP is an innovative healthcare initiative designed to deliver specialist therapeutic infusions (e.g. anti-TNF inhibitors, tocilizumab, zoledronic acid) and subcutaneous injections (e.g. anti-TNF inhibitors, denosumab) within community settings by providing direct HTS and empowering primary care providers (General Practitioners and Polyclinics) to offer these treatments. It aims to address several challenges faced by patients and by healthcare systems: long waiting times for procedures, inconvenience, higher cost of frequent hospital visits for patients and their caregivers, and the strain on inpatient hospital resources. This observational study assesses the feasibility, patient and caregiver experience, and cost of the HTS.
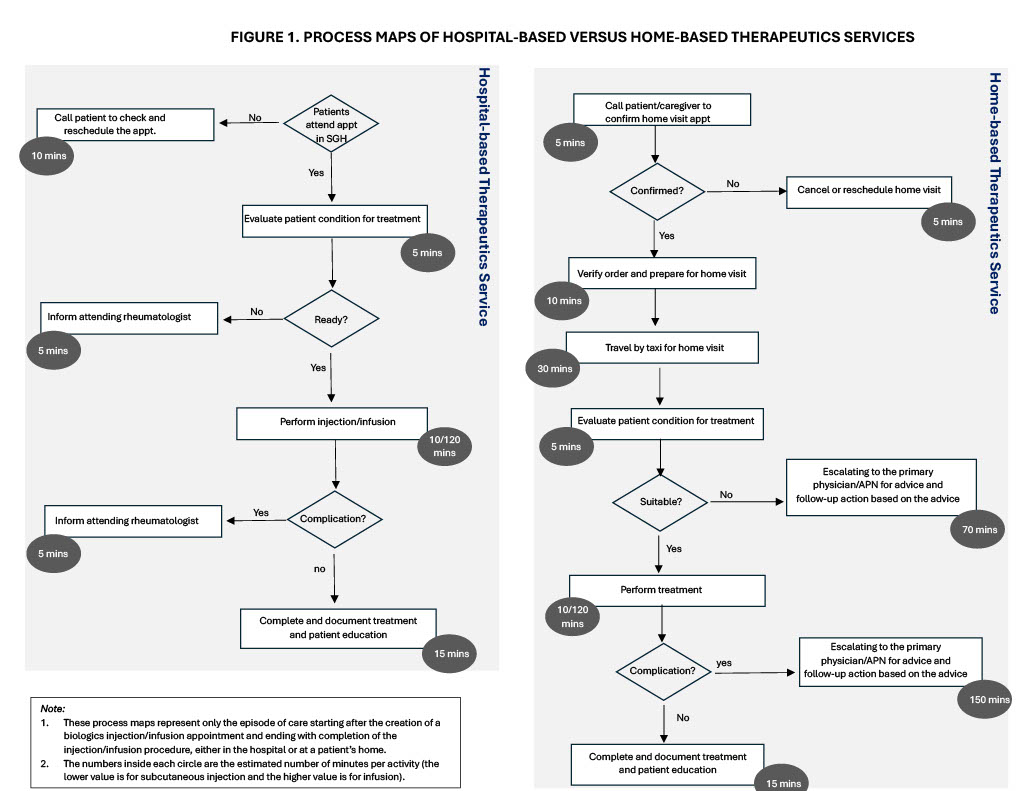
Methods: This evaluation included all patients referred to the HTS by their attending rheumatologists from March 2023 (the start of the CTP) to February 2025. Feasibility was assessed by tracking patients requiring urgent return to hospital-based therapeutics. Patient and caregiver feedback on their experience with the HTS was collected via anonymous online surveys, rating overall experience and specific aspects (convenience, timeliness, scheduling, and comfort) of the HTS. Cost comparison between the HTS and hospital-based care was conducted using time-driven-activity-based-costing (TDABC) method (process maps shown in Figure 1).
Results: The HTS served 230 patients during the evaluation period, administering 867 treatments: 623 subcutaneous injections to 185 patients and 244 therapeutic infusions to 45 patients. No patient needed urgent transfer to hospital-based care. Only three infusions out of the 867 procedures (0.3%) needed rheumatologist consultation: two for pre-infusion stabilisation of blood pressure and one for post-infusion monitoring of side effects that resolved within three days. We received 258 completed service evaluation survey responses (223 by patients and 35 by caregivers), covering 111 intravenous infusion and 147 subcutaneous injection procedures, respectively. The feedback was overwhelmingly positive with 95-98% of respondents agreeing that HTS was convenient, timely, comfortable, and easy to arrange. Cost analysis using TDABC revealed significant savings: the average HTS cost was SGD136 for a subcutaneous injection and SGD260 for a therapeutic infusion, compared to SGD286 and SGD915 respectively for hospital-based alternatives. This translated into total savings of SGD253,270 (SGD93,450 from 623 injections and SGD159,820 from 244 infusions). Even in the three rare cases requiring rheumatologist consultation, the average cost of an infusion in HTS remained more than SGD300 cheaper than that of a hospital-based infusion.
Conclusion: HTS is a safe and less costly service compared to hospital-based therapeutics with overwhelming satisfaction from both patients and caregivers.
To cite this abstract in AMA style:
Xin X, Low A, Yee S, Liu M, Tan C, Ng D, Yeo S. Evaluation of a Home-based Therapeutics Service: Feasibility, Patient and Caregiver Experience, and Cost [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-a-home-based-therapeutics-service-feasibility-patient-and-caregiver-experience-and-cost/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-a-home-based-therapeutics-service-feasibility-patient-and-caregiver-experience-and-cost/