Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Methotrexate (MTX) in combination with the tumor necrosis factor inhibitor adalimumab (ADA) is an effective therapy for treating rheumatoid arthritis (RA), but the minimum dose of MTX in combination with ADA that can achieve an acceptable clinical response is not known.
Objectives: To evaluate different MTX doses in combination with ADA in achieving clinically meaningful improvement in patient-reported outcomes (PROs) in patients with early RA.
Methods: CONCERTO was a 26-week, phase 3, double-blind, parallel-arm study in MTX-naïve patients with active RA <1 year in duration. Patients were randomized 1:1:1:1 to open-label ADA 40 mg every other week plus blinded weekly oral MTX in a dose of 2.5, 5, 10, or 20 mg. Patients in the 20 mg arm started with 10 mg MTX with bi-weekly escalation of 2.5 mg through wk 8. PROs measured at weeks 8 and 26 included the following: Health Assessment Questionnaire Disability Index (HAQ-DI); patient global assessment of disease activity (PGA); 36-item Short-Form Health Survey, Version 2 (SF-36v2), Physical Component Summary (PCS) and Mental Component Summary (MCS); Medical Outcomes Study Sleep Scale (MOS SS); Compliance Questionnaire Rheumatology (CQR); and Treatment Satisfaction Questionnaire for Medication (TSQM). Differences in mean changes in PROs among the 4 treatment groups were analyzed using mixed models. No adjustments for multiplicity were applied.
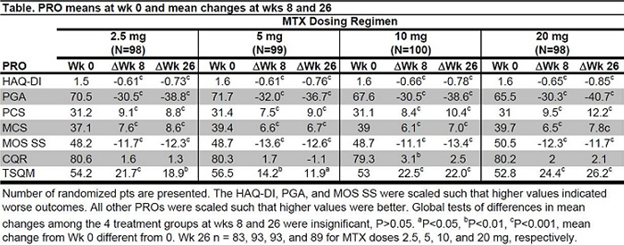
Results: Of the 395 patients included in the intent-to-treat population, 358 (91%) completed 26 weeks of treatment; 15, 7, 6, and 9% of pts receiving 2.5, 5, 10, or 20 mg MTX, respectively, discontinued treatment prior to completion. Clinically and statistically significant improvements from week 0 in most PROs occurred in all 4 dose groups by 8 weeks, were maintained through 26 weeks, and were similar regardless of MTX dose with no statistically significant difference between the dose groups with respect to the HAQ-DI, PCS, and MCS.
Image/graph:
Conclusion: Patients treated with ADA in combination with MTX 2.5, 5, 10 or 20 mg per week experience similar improvements in PROs by week 8 that are maintained through 26 wks. The differences associated with MTX dose are not statistically significant and, in general, not clinically meaningful.
Disclosure:
R. Fleischmann,
AbbVie, Pfizer, Merck, Roche, UCB, Celgene, Amgen, Astra-Zeneca, BMS, Janssen, Lilly, and Novartis,
2,
AbbVie, Pfizer, Merck, Roche, UCB, Celgene, Amgen, Astra-Zeneca, BMS, Janssen, Lilly, and Novartis,
5;
A. Kivitz,
AbbVie, Amgen, AstraZeneca, BMS, Celgene, Genentech, Janssen, Pfizer, and UCB,
2,
BMS, Genentech, UCB, AbbVie, Pfizer,
5,
Pfizer, BMS,
8;
R. F. van Vollenhoven,
AbbVie, BMS, GSK, Merck, Pfizer, Roche, and UCB,
2,
AbbVie, BMS, GSK, Merck, Pfizer, Roche, Lilly, Vertex and UCB,
5;
J. W. Shaw,
BMS,
3,
AbbVie,
9;
S. Florentinus,
AbbVie,
1,
AbbVie,
3;
P. M. Karunaratne,
AbbVie,
1,
AbbVie,
3;
H. Kupper,
AbbVie,
1,
AbbVie,
3;
M. Dougados,
AbbVie, BMS, Merck, Novartis, Pfizer, Sanofi-Aventis, and UCB,
2,
AbbVie, BMS, Merck, Novartis, Pfizer, Sanofi-Aventis, and UCB,
5;
G. Burmester,
AbbVie, BMS, MSD, Pfizer, Roche, and UCB,
2,
AbbVie, BMS, MSD, Pfizer, Roche and UCB,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/no-differences-in-patient-reported-outcomes-by-methotrexate-dose-among-early-rheumatoid-arthritis-patients-treated-concomitantly-with-adalimumab-results-from-the-concerto-trial/