Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
It is estimated that between 10 and 30% of rheumatoid arthritis (RA) patients are methotrexate (MTX)-intolerant and discontinuation is common in clinical practice. For RA patients’ requiring biological treatment and not tolerating MTX, the options are either combination therapy with other disease-modifying antirheumatic drugs (DMARDs) or biological monotherapy. In Denmark it is mandatory to register the patients in the national database, DANBIO. The objective of the present study was to elucidate the prevalence of Danish RA patients currently on biological monotherapy.
Methods:
All RA patients, treated with biologics, that were registered in DANBIO, as receiving biological treatment as monotherapy during the period May 1st 2011 to April 30th 2013 were eligible for inclusion. All files of individual patients were reviewed for inconsistence with the treatment registration in DANBIO. Results are presented as descriptive statistics, with no adjustments or statistical models applied.
Results:
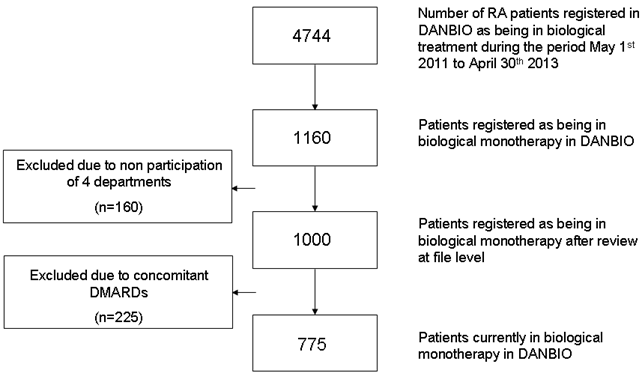
Twenty-one of 25 Danish rheumatology departments participated in the study. In total 4,744 RA patients were registered in DANBIO as being in biological treatment, of which 4,151 (87.5%, Figure) were treated in the participating departments. Of these, 1,000 (24.1%) were registered as receiving biological monotherapy. 225 (22.5%) of the 1000 were excluded after file review due to use of concomitant conventional DMARDs not registered in DANBIO, leaving 775 (18.7%) in verified currently biological monotherapy. Etanercept, adalimumab, tocilizumab and rituximab were the biological drugs that most often were given in monotherapy (Table).
Conclusion:
In Denmark, 18.7% of biological treatments for RA are prescribed as monotherapy, i.e. without concomitant DMARDS. Monotherapy is prevalent across all biologics, while with a preponderance with adalimumab, etanercept, and tocilizumab, all three having the indication monotherapy.
Acknowledgement:
This study was supported by the Oak Foundation and Roche Denmark
Figure: Flow diagram
Table: The distribution of various biologics.
|
|
Numbers treated (N=775) |
% of total |
|
17 |
2.2 |
|
|
Adalimumab |
165 |
21.3 |
|
Anakinra |
3 |
0.4 |
|
Certolizumab |
26 |
3.4 |
|
Etanercept |
284 |
36.6 |
|
Golimumab |
21 |
2.7 |
|
Infliximab |
48 |
6.2 |
|
Rituximab |
92 |
11.9 |
|
Tocilizumab |
119 |
15.3 |
Disclosure:
T. S. Joergensen,
Roche Pharmaceuticals,
2;
L. E. Kristensen,
Pfizer, Wyeth, Abbott, BMS, MSD and Schering-Plough.,
5;
T. Lorenzen,
Advisory Board member of Roche and Pfizer,
6;
J. Jensen,
None;
L. Zanjani,
None;
T. Laursen,
None;
S. Butt,
None;
M. Y. Dam,
None;
H. M. Lindegaard,
Lilly, MSD, Nordpharma and Roche,
5;
J. Espesen,
None;
O. Hendricks,
None;
P. Kumar,
None;
A. Kincses,
None;
L. H. Larsen,
None;
M. Andersen,
None;
E. K. Næser,
None;
D. V. Jensen,
None;
J. Grydehøj,
None;
B. Unger,
None;
N. Dufour,
None;
V. N. Sørensen,
None;
S. Vildhøj,
None;
I. M. J. Hansen,
None;
J. Raun,
None;
M. L. Hetland,
Pfizer, ROche og MSD.,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-prevalence-of-biological-monotherapy-among-rheumatoid-arthritis-patients-in-denmark-results-from-the-danish-nationwide-danbio-registry/