Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Zasocitinib (TAK-279) is an oral, selective TYK2 inhibitor under investigation for the treatment of immune-mediated inflammatory diseases including psoriatic arthritis (PsA). In a phase 2b trial in patients with active PsA (NCT05153148), 12-week ACR20 response rates were significantly greater among patients receiving zasocitinib 15 or 30 mg once daily (QD) than those receiving placebo (PBO, primary endpoint; p=0.002).1 PsA treat-to-target recommendations state that the treatment target should be remission or low disease activity (LDA);2 here, additional disease activity data are presented.
Methods: This was a phase 2b, randomized, multicenter, double-blind, placebo-controlled, multiple-dose study. Patients (18–70 years) with active PsA were randomized 1:1:1:1 to receive oral zasocitinib 5, 15 or 30 mg QD or PBO for 12 weeks.1 Secondary endpoints included change from baseline (BL) to Week 12 in Disease Activity Index for PsA (DAPSA). Exploratory endpoints included changes from BL to Week 12 in PsA Disease Activity Score (PASDAS) and Disease Activity Score 28 with high-sensitivity C-reactive protein (DAS28-hsCRP), and the proportion of patients achieving improvements in PsA Response Criteria (PsARC) at Week 12. Differences were assessed using a Mantel–Haenszel test (binary endpoints) and a mixed model for repeated measures (continuous endpoints). All p values are nominal.
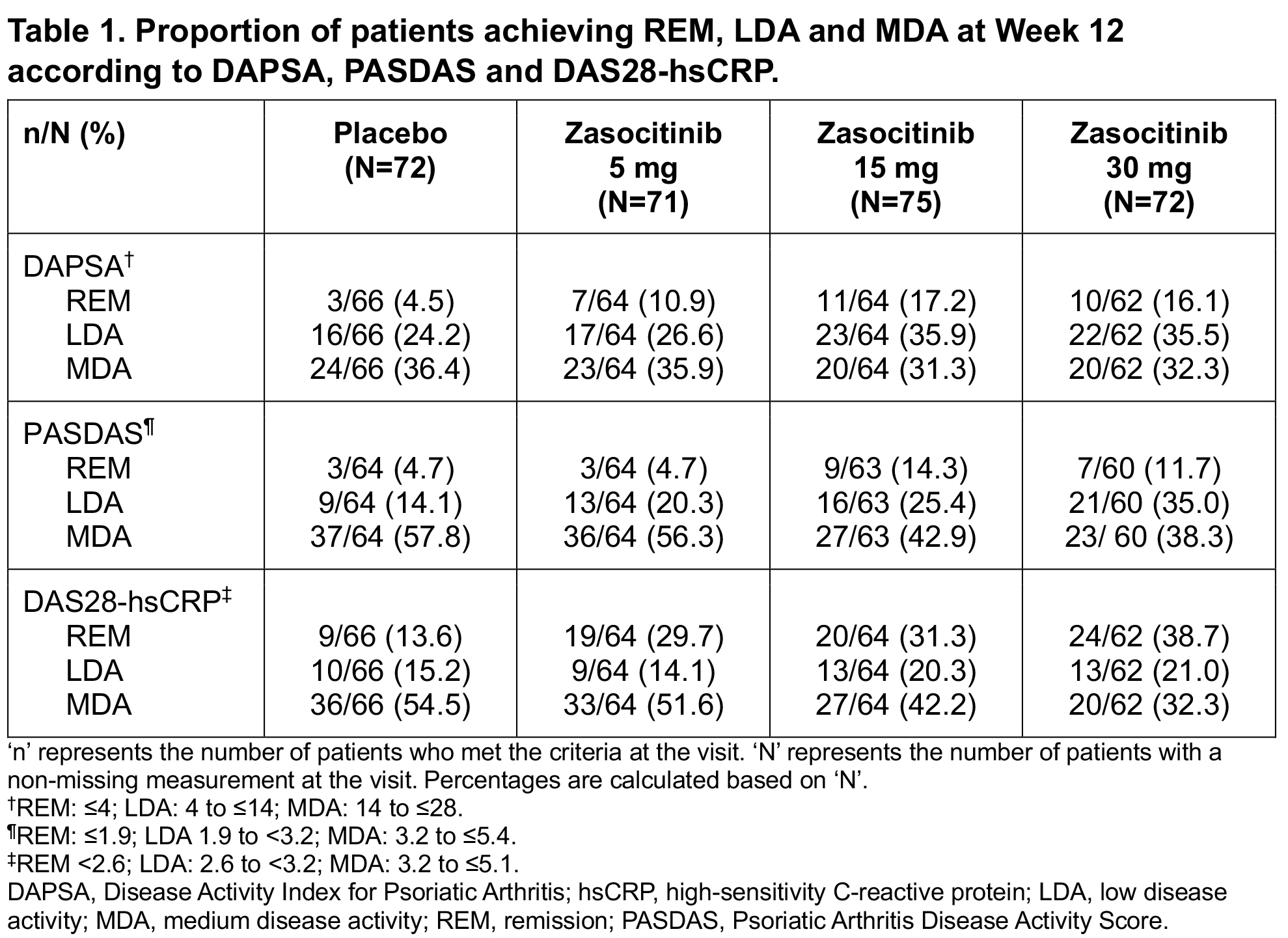
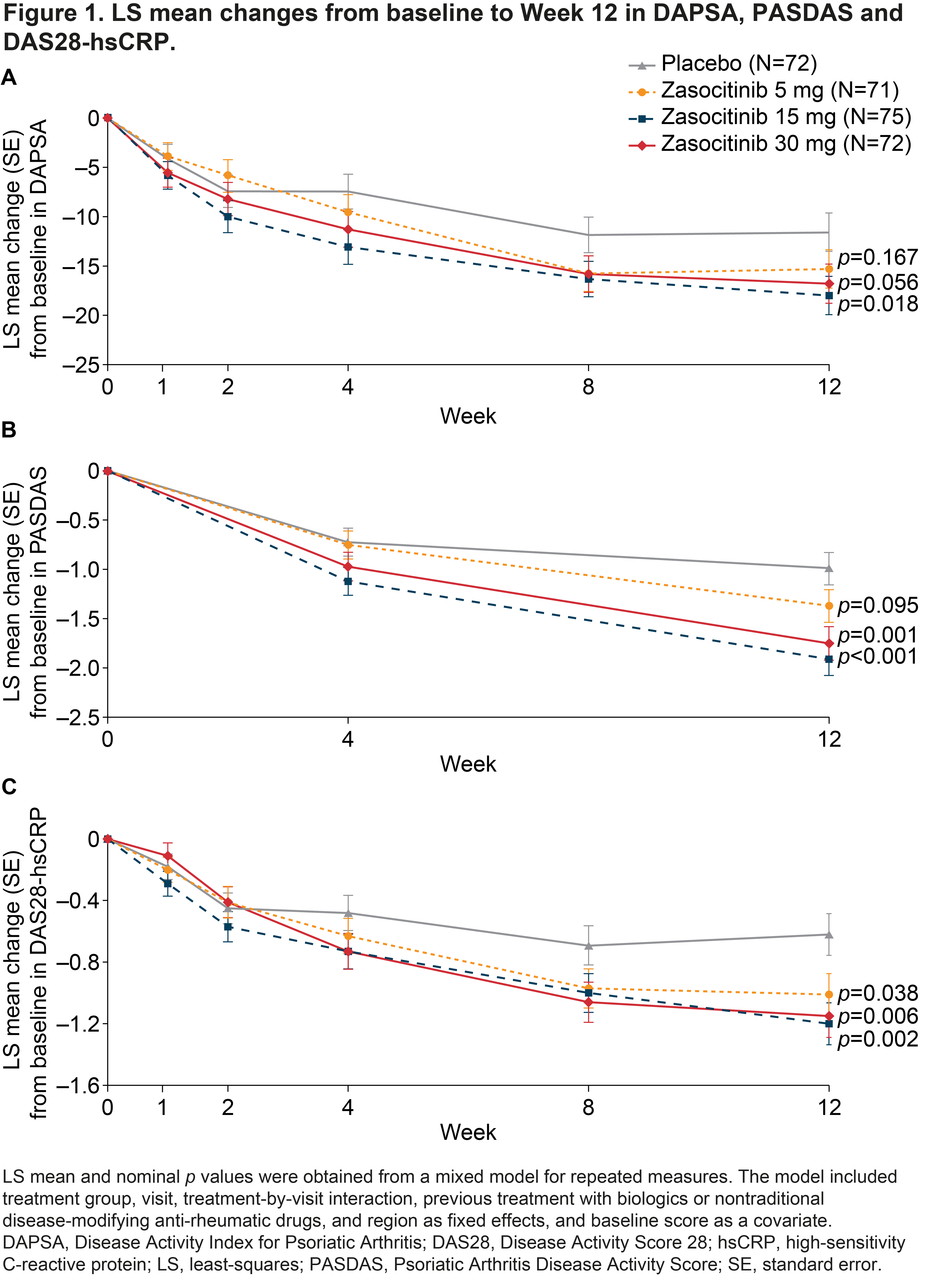
Results: In total, 290 patients were randomized and treated. BL disease characteristics were generally comparable across groups; mean (standard deviation; SD) PsA disease duration was 6.9 (7.1) years and mean hsCRP level was 7.0 (12.2) mg/L. Mean (SD) tender joint count and DAPSA at BL were lower in the zasocitinib 30 mg arm (14.0 [9.4] and 33.4 [15.8], respectively) than in the PBO (18.5 [14.7] and 38.3 [20.7], respectively), zasocitinib 5 mg (18.4 [15.2] and 39.0 [21.6], respectively) and zasocitinib 15 mg (18.0 [12.8] and 40.1 [18.0], respectively) arms. Greater numbers of patients receiving zasocitinib 15 or 30 mg than those receiving PBO or zasocitinib 5 mg were considered in remission or achieved LDA at Week 12, as measured using DAPSA, PASDAS and DAS28-hsCRP (Table 1). Least-squares mean changes from BL to Week 12 in DAPSA, PASDAS and DAS28-hsCRP were generally greater in patients receiving zasocitinib than in those receiving PBO, at all doses tested (Figure 1). Compared with PBO, patients receiving zasocitinib 15 or 30 mg had greater reductions in DAPSA (–17.99 [p=0.018] and –16.79 [p=0.056], respectively), PASDAS (–1.91 [p< 0.001] and –1.75 [p=0.001], respectively) and DAS28-hsCRP (–1.20 [p=0.002] and –1.15 [p=0.006], respectively) at Week 12. At Week 12, a numerically higher proportion of patients receiving zasocitinib 15 mg or 30 mg achieved a PsARC response versus those receiving PBO (p=0.024 and p=0.140, respectively).
Conclusion: Treatment with zasocitinib 15 and 30 mg resulted in higher rates of remission or LDA after 12 weeks in patients with PsA compared with placebo. Zasocitinib shows promise as a novel oral therapy in PsA and warrants further investigation in phase 3 trials.
References:
1. Kivitz A et al. Arthritis Rheumatol 2023;75 (Suppl 9).
2. Gossec L et al. Ann Rheum Dis 2024;83:706–19.
To cite this abstract in AMA style:
Mease P, Kivitz A, Muensterman E, Lertratanakul A, Hong T, Chen J, Mark E, Baraliakos X. Zasocitinib (TAK-279), an Oral, Selective Tyrosine Kinase 2 Inhibitor: Achievement of Remission and Additional Improvements in Disease Activity in Patients with Psoriatic Arthritis Enrolled in a Phase 2b Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/zasocitinib-tak-279-an-oral-selective-tyrosine-kinase-2-inhibitor-achievement-of-remission-and-additional-improvements-in-disease-activity-in-patients-with-psoriatic-arthritis-enrolled-in-a-phase/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/zasocitinib-tak-279-an-oral-selective-tyrosine-kinase-2-inhibitor-achievement-of-remission-and-additional-improvements-in-disease-activity-in-patients-with-psoriatic-arthritis-enrolled-in-a-phase/