Session Information
Date: Monday, November 18, 2024
Title: Abstracts: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: CT-P41 is a proposed biosimilar of the reference denosumab (DEN), a fully human monoclonal antibody that binds the cytokine receptor activator of NF-κb ligand (RANKL). In this phase 3 study, the therapeutic equivalence of efficacy as assessed by changes from baseline in lumbar spine bone mineral density (BMD) at Week 52 and the similarity of pharmacodynamics as assessed by changes in marker of bone turnover up to Week 26 for CT-P41 versus DEN were demonstrated in postmenopausal women with osteoporosis (PMO) (WCO-IOF-ESCEO Congress 2024; abstract number P683, P684). Here, we report the additional efficacy and safety results from Week 52 to Week 78 including switching data from DEN to the CT-P41 (Treatment Period [TP] II).
Methods: At Week 0, patients were randomly assigned in a 1:1 ratio to receive 60 mg of CT-P41 or DEN every 6 months (Weeks 0 and 26; TP I). Before dosing at Week 52, patients initially assigned to DEN in TP I were re-randomized in a 1:1 ratio to continue DEN or to switch to CT-P41 in TP II. Patients assigned to CT-P41 in TP I continued treatment with CT-P41. Efficacy and safety were evaluated up to Week 78.
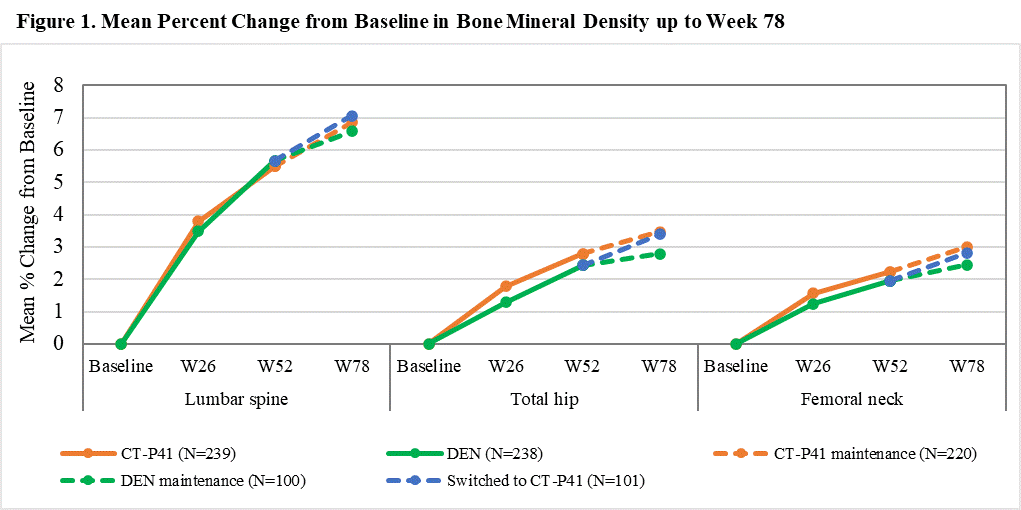
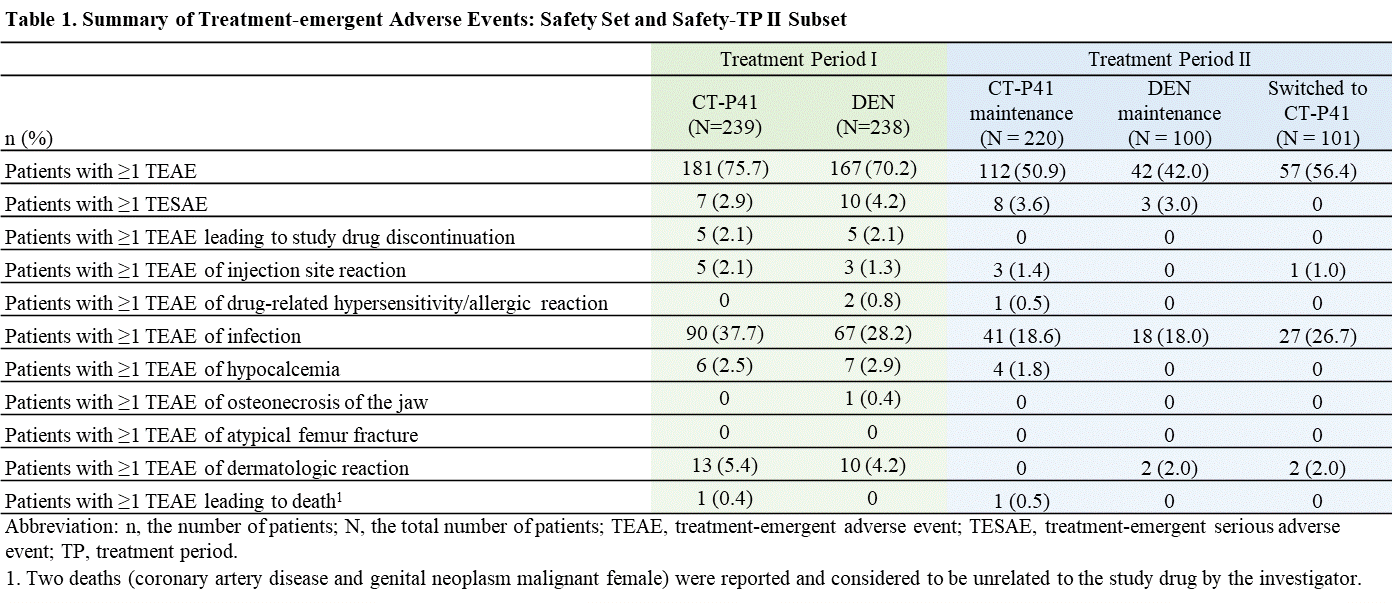
Results: In TP II, 422 patients were randomized at Week 52 (CT-P41 maintenance group: 221, DEN maintenance group: 100, Switched to CT-P41 group: 101). The mean percent change from baseline in the BMD for the lumbar spine, total hip, and femoral neck increased until Week 78 and was comparable among the groups (Figure 1). A new vertebral fracture was reported in 1 (0.5%) patient in the CT-P41 maintenance group at Week 78. Nonvertebral fractures were reported in 2 (0.9%) patients in the CT-P41 maintenance group and 1 (1.0%) patient in the switched to CT-P41 group. No hip fracture was reported. No notable safety issue was observed following a transition from DEN to CT-P41 compared with CT-P41 maintenance and DEN maintenance groups. In total, 211/421 (50.1%) patients experienced at least 1 treatment-emergent adverse event (TEAE) (112/220 [50.9%], 42/100 [42.0%], and 57/101 [56.4%] patients in the CT-P41 maintenance, DEN maintenance, and switched to CT-P41 groups, respectively) and most of them were Grade 1 or 2 in severity. Overall, 11 (2.6%) patients experienced at least 1 serious TEAE (8/220 [3.6%], 3/100 [3.0%], and 0/101 patients in the CT-P41 maintenance, DEN maintenance, and switched to CT-P41 groups, respectively). Hypocalcaemia was reported in 4 (1.8%) patients only in the CT-P41 maintenance group (all cases were non-serious). There was one death owing to a TEAE of “genital neoplasm malignant female” in the CT-P41 maintenance group; this was considered unrelated to the study drug by the investigator (Table 1).
Conclusion: The comparable and sustained efficacy results up to Week 78 supported the therapeutic equivalence of CT-P41 and DEN in treating PMO. CT-P41 was also well tolerated with a safety profile comparable to that of DEN, and no notable safety issue was identified following the single transition from DEN to CT-P41 compared with CT-P41 and DEN maintenance groups up to Week 78.
To cite this abstract in AMA style:
Reginster J, Silverman S, Czerwinski E, Borowy P, Budlewski T, Kwiatek J, Postol S, Põder A, Supronik J, Kim S, Suh J, Yang G, Han N, Kim N, Bae S. Efficacy and Safety Results of CT-P41 (Proposed Denosumab Biosimilar) Compared to Reference Denosumab in Postmenopausal Women with Osteoporosis: 78-Week Results from Phase 3 Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-results-of-ct-p41-proposed-denosumab-biosimilar-compared-to-reference-denosumab-in-postmenopausal-women-with-osteoporosis-78-week-results-from-phase-3-randomized-controlled-tria/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-results-of-ct-p41-proposed-denosumab-biosimilar-compared-to-reference-denosumab-in-postmenopausal-women-with-osteoporosis-78-week-results-from-phase-3-randomized-controlled-tria/