Session Information
Date: Monday, November 18, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The aim of this prospective cohort study is to evaluate the efficacy and safety of Janus kinase (JAK) inhibitor upadacitinib in refractory Behçet’s Syndrome (BS).
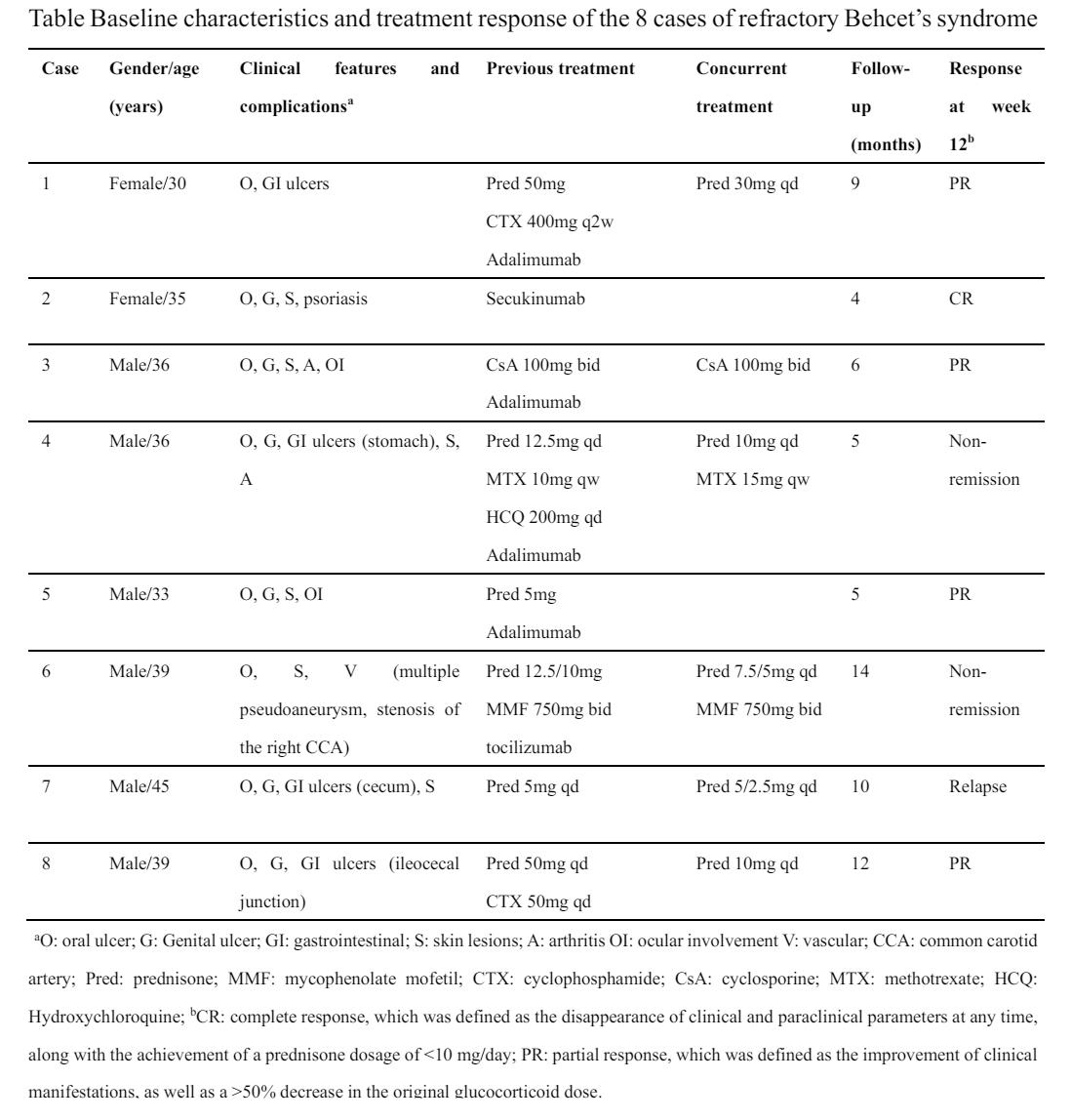
Methods: In this multi-center, prospective open-label pilot trial, we enrolled 8 patients diagnosed with BS in Peking University People’s Hospital, Shijiazhuang People’s Hospital, and Qingdao Women and Children’s Hospital from June 2023 to February 2024, fulfilling the 2014 International Criteria for Behçet’s Disease. Refractory BS is defined as unresponsiveness to systemic treatment and biologic agents for at least 12 weeks’ duration. All patients received upadacitinib 15 mg once a day, with background treatment of immunosuppressor and glucocorticoid. All patients were followed for more than 12 weeks. Every 4 weeks, patients visited the hospital, and then the medical team assessed disease activity and adverse events. The clinical response was evaluated by the change of BDCAF score at week 12. Complete response (CR) was defined as the disappearance of clinical and paraclinical parameters at any time, along with the achievement of a prednisone dosage of < 10 mg/day; partial response (PR) was defined as the improvement of clinical manifestations, as well as a >50% decrease in the original glucocorticoid dose.
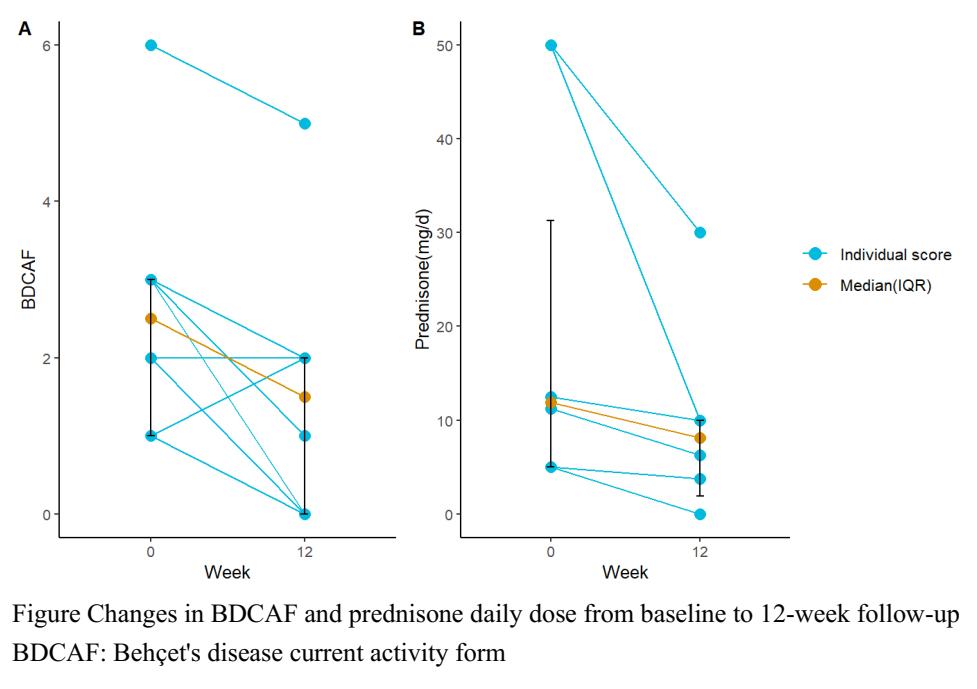
Results: The Demographic data and clinical features of the eight patients are described in Table. In our cohort, the male-to-female ratio of cases was 3:1, with a mean age of 36.6±4.5 years. Disease activity assessed by BDCAF score significant decreased after treatment of upadacitinib (2.5(IQR 1-3) vs 1.5 (IQR 0-2), p=0.047) (Figure A). One patient achieved CR and 4 patients achieved PR. One patient suffered a relapse after achieving PR. In addition, patients who received glucocorticoids as background treatment tapered their baseline glucocorticoid dose (11.9(IQR 5.0-31.3) vs 8.1 (IQR 1.9-10.0), p=0.027, converted to prednisone) (Figure B). During the follow-up visit, except for one patient who suffered from joint pain, no adverse events were reported among the other patients in our study.
Conclusion: Our result suggests the efficacy, safety and steroid-sparing effect of upadacitinib in refractory BS. Upadacitinib is a promising therapeutic option in treating refractory BS.
To cite this abstract in AMA style:
Xia W, Li J, Liu T, Li Y. Upadacitinib for Refractory Behçet’s Syndrome: A Multi-center, Prospective, Open-label, Pilot Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/upadacitinib-for-refractory-behcets-syndrome-a-multi-center-prospective-open-label-pilot-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-for-refractory-behcets-syndrome-a-multi-center-prospective-open-label-pilot-study/