Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Anifrolumab is a biological treatment with recent approval for systemic lupus erythematosus (SLE), supported by its efficacy in clinical trials. However, data from real clinical practice are lacking. The purpose of this work is to describe patient’s baseline characteristics at the time of anifrolumab initiation, reasons for its prescription, and efficacy and adverse effects at 3 months in patients with SLE under real-life conditions followed up in Spanish rheumatology departments.
Methods: Observational, uncontrolled, ambispective and multicenter study of a cohort of patients with SLE (according to the 2019 EULAR/ACR criteria), treated with anifrolumab in 28 rheumatology departments. Patients who received at least one dose of anifrolumab were included. Sociodemographic, clinical, blood test variables and treatments, were collected. In addition, indexes or scales of activity (SLEDAI-2k, SFI, SLE-DAS, CLASI), damage (SLICC/ACR/DI), fatigue (FACIT), neuro-cognitive function (MoCA) and disease impact (LIT) at the baseline visit were calculated. The efficacy and security analysis comparing the basal and 3-month visit post initiation of anifrolumab were performed using a McNemar test for paired categorical samples for the categorical variables and a sign test for the continuous variables. Ethics Committee code: 2023/10814.
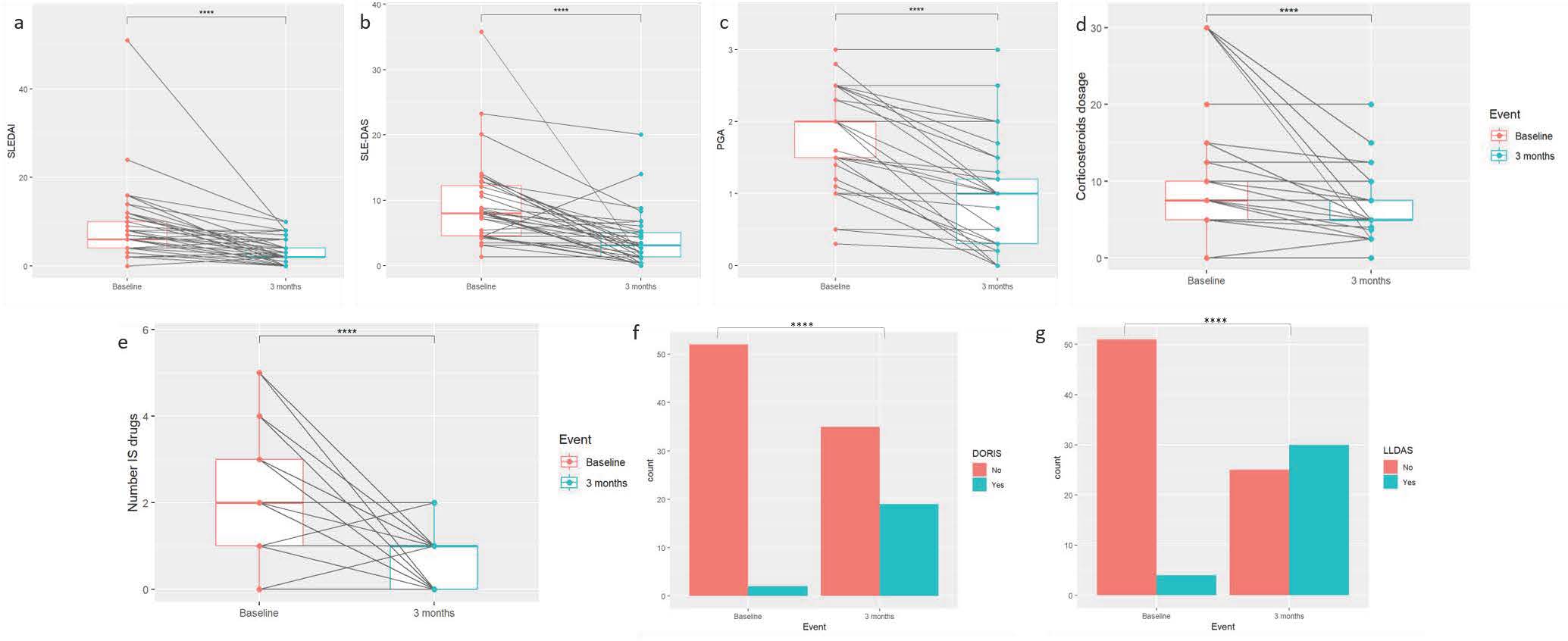
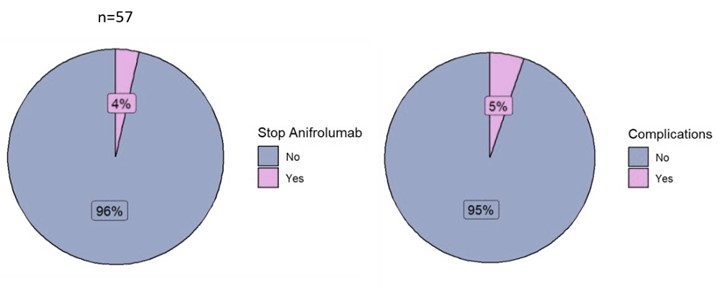
Results: 105 SLE patients were included. Table 1 shows the baseline demographic, clinical characteristics, reasons for anifrolumab initiation and treatments of the patients. We found a statistically significant difference in the SLEDAI, SLEDAS, physician global assessment, glucocorticoids dose, number of concomitant immunosuppressant drugs used, and the percentage of patients in remission (DORIS-21) or in low disease activity (LLDAS) 3 months after initiation of anifrolumab (n=61) vs the basal visit (See Figure 1). 11/59 (18.6%) underwent flares (10, 1 flare; 1, 2) during those 3 first months, of which 2 were severe (according to SFI). 40/52 (76.9%) showed a response according to the physician’s global opinion, 40/46 (86.9%) showing a response ≥ 20%, 31/46 ≥ 50% and 22/51 (43.1%) serological response. 2/57 (3.5%) patients discontinued anifrolumab due to adverse effects (1 pneumonia, 1 dyspnea) and 3 had to be hospitalized (1 serious respiratory infection, 1 SLE activity, 1 other) (Figure 2).
Conclusion: Anifrolumab was initiated in a real-life setting in patients with a long disease course and refractory to several treatments, with a predominance of mucocutaneous and joint involvement. Anifrolumab showed a significant improvement in different efficacy variables 3 months after initiation, with a relevant percentage reaching LLDAS or DORIS remission, and with barely any security events. This indicates that anifrolumab seems to be even more effective when used in real-life clinical practice as compared to clinical trials.
To cite this abstract in AMA style:
Carrión-Barberà I, Salman Monte: T, Triginer L, García-Villanueva M, Garrote Corral S, Riveros Frutos A, Font J, Galindo-Izquierdo M, de Frías Polo C, Fragío Gil J, Moriano C, Díez Álvarez E, Bernabéu P, Vela-Casasempere P, Cortés-Hernández J, Sandoval Moreno S, Narvaez-García J, Hernández-Martín A, Paula Magallares B, Piqueras García M, Marras C, Ramos C, Gomez-Puerta J, Frade-Sosa B, Fernández-Ortiz A, Garcia-Aparicio A, Altabás-González I, Hernández-Baldizón S, Ros-Vilamajó I, García Cirera S, Garijo Bufort M, Sala L, Martínez-Barrio J, Rosas-Gómez de Salazar J, Heredia S, Torrente-Segarra V, Brandy-Garcia A, del Val del amo N, Fito-Manteca C, Calvo-Alen J, Pego-Reigosa J, Rúa-Figueroa Í. Ambispective, Multicenter Registry of Treatment with Anifrolumab in Real Life in Patients with Systemic Lupus Erythematosus from Spanish Rheumatology Departments (ANIFRO-Reu): Efficacy, Safety and Patient’s Characteristics [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/ambispective-multicenter-registry-of-treatment-with-anifrolumab-in-real-life-in-patients-with-systemic-lupus-erythematosus-from-spanish-rheumatology-departments-anifro-reu-efficacy-safety-and-pat/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ambispective-multicenter-registry-of-treatment-with-anifrolumab-in-real-life-in-patients-with-systemic-lupus-erythematosus-from-spanish-rheumatology-departments-anifro-reu-efficacy-safety-and-pat/