Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: In the FOREMOST study of early oligoarticular psoriatic arthritis (PsA), fewer patients (pts) receiving apremilast (APR) progressed from ≤4 active joints (all joints; oligoarthritis) to >4 (polyarthritis) over 16 weeks compared with placebo (PBO). FOREMOST offers a unique opportunity to identify baseline characteristics associated with disease progression from ≤4 active joints to >4 joints, as well as the ability of APR to impact disease progression.
Methods: FOREMOST (NCT03747939), a multicenter, randomized, double-blind, PBO-controlled trial, included pts with early PsA (duration ≤5 years) and limited joint involvement ( >1–≤4 swollen and >1–≤4 tender joint count [SJC and TJC]; 66–68 joints assessed). Pts were randomized 2:1 to APR (30 mg BID) or PBO for 24 weeks (early escape at Week [W] 16), followed by an extension phase in which all pts received APR through W48. This post hoc analysis assessed the percentage of pts with an active (swollen and/or tender) joint count ≤4 at baseline who progressed to an active joint count >4 at W16 stratified by baseline characteristics. Clinically relevant demographic/disease characteristics with reasonable sample size were selected from all available baseline data. Data are reported as observed.
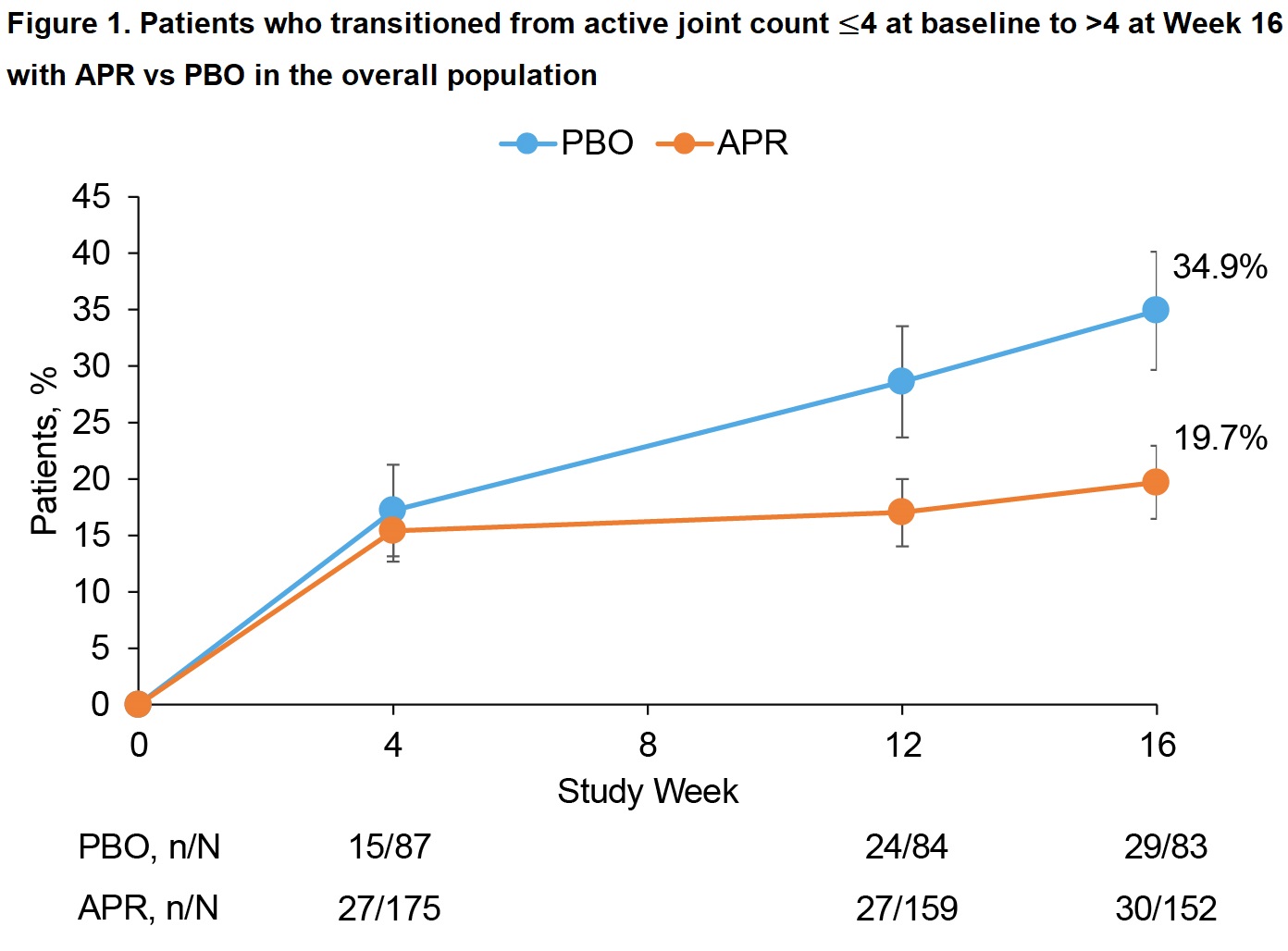
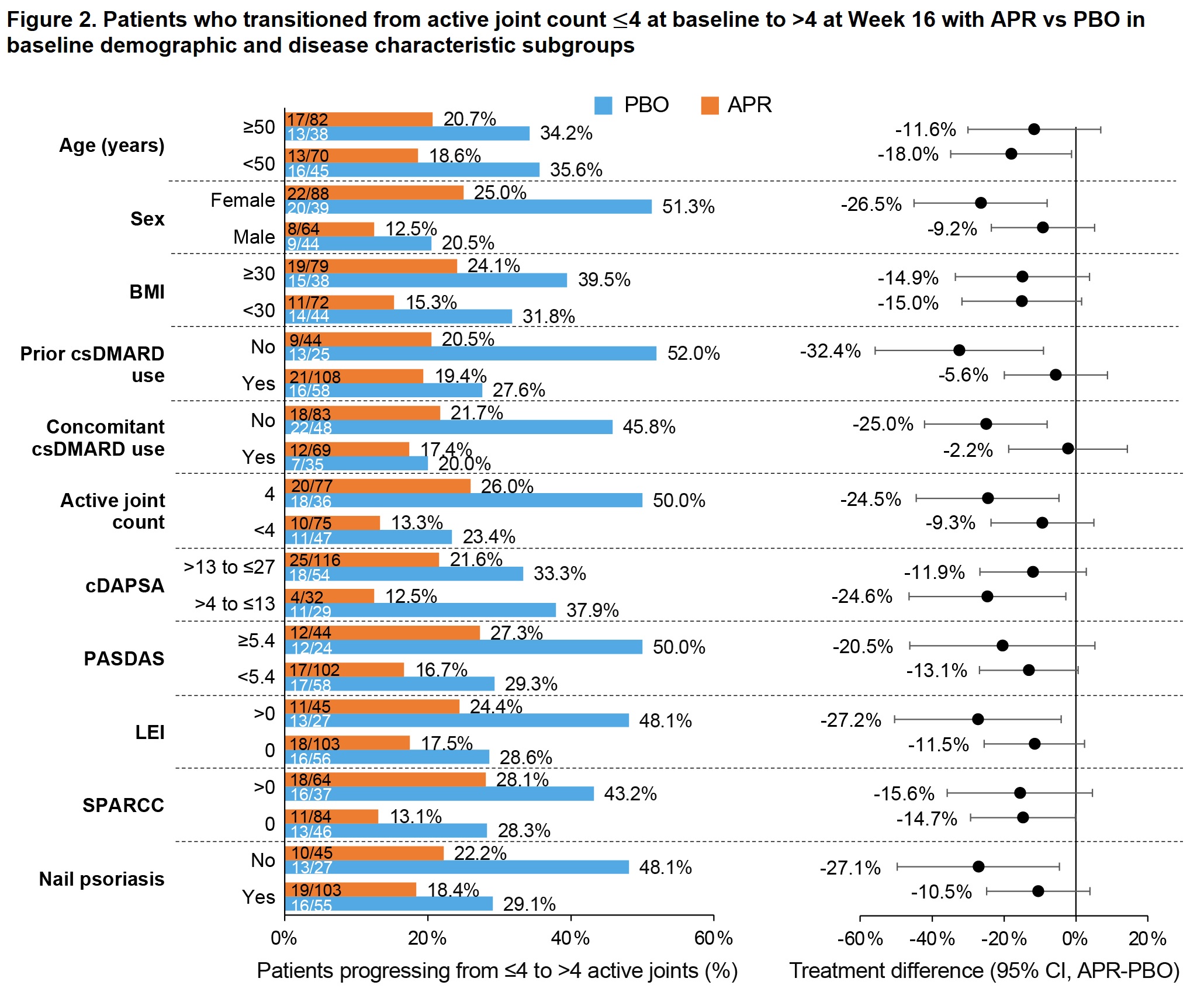
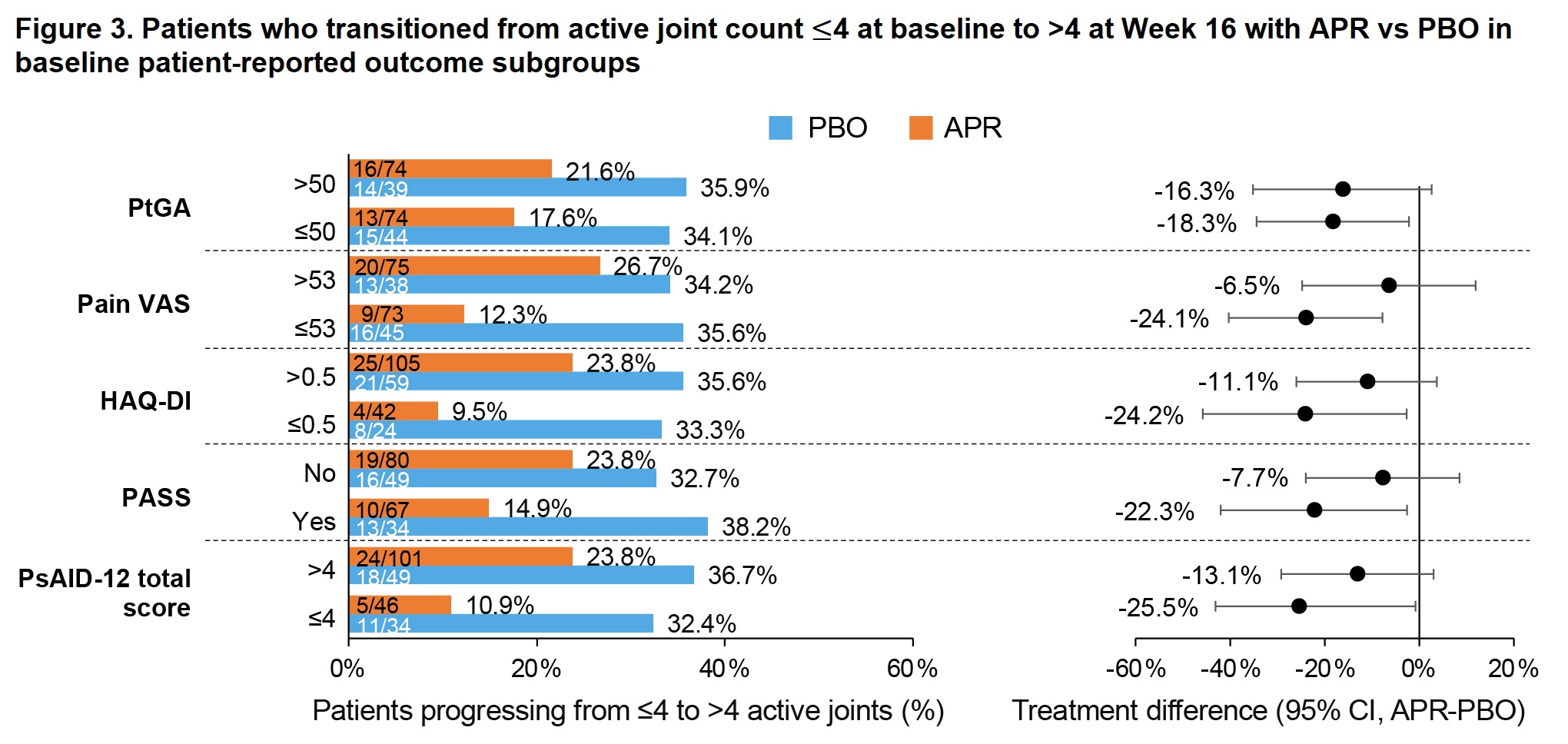
Results: Of 308 pts randomized (APR, n=203; PBO, n=105), most (268/308 [87%]) had ≤4 active joints at baseline (APR, n=176; PBO, n=92). Baseline demographics/disease characteristics were generally similar between the APR and PBO groups, with the exception of a higher proportion of females in the APR group (58.5% vs 48.9%). Compared with APR, a higher proportion of pts in the PBO group progressed to >4 active joints at W16 (34.9% vs 19.7%; treatment difference: -14.8%) (previously published; Figure 1). This trend was consistent across baseline demographic/disease characteristic subgroups (Figures 2 and 3). Female pts were more likely to progress to >4 active joints than male pts (APR, 25.0% vs 12.5%; PBO, 51.3% vs 20.5%), and a greater treatment difference was observed in females (-26.5 vs -9.2; Figure 2). Treatment differences were consistent regardless of baseline body mass index (Figure 2). Greater treatment differences were observed in pts who had not previously or were not currently using conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) compared with those who were (Figure 2). Pts with enthesitis at baseline were more likely to progress to >4 active joints than those without (Figure 2), with a treatment difference observed when enthesitis was measured by Leeds Enthesitis Index (LEI; -27.2% vs -11.5%) but not when measured by Spondyloarthritis Research Consortium of Canada Index (SPARCC; -15.6% vs -14.7%). Greater treatment differences were seen in pts without nail psoriasis versus those with nail psoriasis (-27.1% vs -10.5%) (Figure 2).
Conclusion: In FOREMOST, being female, having more active joints, or having enthesitis were identified as demographic/disease characteristics associated with disease progression from ≤4 to >4 active joints in early oligoarticular PsA. APR reduced disease progression after 16 weeks compared with PBO, with larger treatment differences observed across a number of demographic/disease characteristics.
cDAPSA scores >4 to ≤13 indicate low disease activity; scores >13 to ≤27 indicate moderate disease activity. PASDAS scores ≥5.4 indicate high disease activity.
APR, aremilast; BMI, body mass index; cDAPSA, clinical Disease Activity in Psoriatic Arthritis; CI, confidence interval; csDMARD, conventional synthetic disease-modifying antirheumatic drug; LEI, Leeds Enthesitis Index; PASDAS, Psoriatic Arthritis Disease Activity Score; PBO, placebo; SPARCC, Spondyloarthritis Research Consortium of Canada Index.
HAQ-DI, Health Assessment Questionnaire-Disability Index; PASS, Patient-Acceptable Symptom State; PsAID_12, Psoriatic Arthritis Impact of Disease 12-Item; PtGA, Patient Global Assessment of Disease Activity; VAS, visual analog scale.
To cite this abstract in AMA style:
Mease P, Coates L, Ogdie A, Gladman D, Zabotti A, Baraliakos X, Deignan C, Jardon S, Wang R, Teng L, Gossec L. Impact of Baseline Factors on Disease Progression and Apremilast Efficacy in Early Oligoarticular Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-baseline-factors-on-disease-progression-and-apremilast-efficacy-in-early-oligoarticular-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-baseline-factors-on-disease-progression-and-apremilast-efficacy-in-early-oligoarticular-psoriatic-arthritis/