Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Drug survival rates serve as key indicators for assessing both the efficacy and safety of treatments in real life setting. Our aim was to compare the drug retention of first line biologics, anti-tumor necrosis factor (anti-TNF) therapies or anti-interleukin 17 (anti-IL17) therapies.
Methods: This retrospective cohort study, using data from the French National Health Data System (SNDS), included adult patients diagnosed with Spondyloarthritis (SpA), encompassing axial SpA (axSpA) and Psoriatic Arthritis (PsA), who initiated a first biologic, either anti-TNF or anti-IL17 agent, between January 1, 2015, and December 31, 2018. Follow-up extended until December 31, 2020. We choose to discontinue follow-up before the COVID-19 pandemic period, which could have had an impact on drug survival. Drug survival was described with Kaplan-Meier curves, and predictive factors were identified with a multivariable Cox model adjusted for age, gender, calendar initiation period, and potential indication modifiers such as multiple sclerosis, heart failure, psoriasis, and cancer.
Results: Of the 26,992 SpA patients initiating a first anti-TNF (n = 26,039) or a first anti-IL17 (n = 953), with a median age at initiation of 44.5 years ± 13.1 and 56.2% female, 25,553 (95.0%) had a diagnosis of axSpA, and 5,453 (20.2%) had PsA.
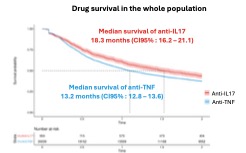
In the entire population, the median drug survival was 13.2 months (CI95 %: 12.8–13.6) for anti-TNF and 18.3 months (CI95 %: 16.2–21.1) for anti-IL17. The retention rates at 1 and 2 years were 52.3% (51.7-53.0) and 37.4% (36.9-38.0) with anti-TNF, compared to 60.6% (57.6-63.8) and 43.6% (40.5-46.9) with anti-IL17.
In the axSpA population, the median drug survival was 13.2 months (CI95 %: 12.9–13.7) for anti-TNF and 18.3 months (CI95 %: 19.0–21.5) for anti-IL17 (adjusted HR = 0.83 [0.76-0.91], p < 0.001). In the PsA population, the median drug survival was 12.0 months (CI95 %: 11.3–12.5) for anti-TNF and 19.7 months (CI95 %: 16.3–24.5) for anti-IL17 (adjusted HR = 0.73 [0.63-0.83], p < 0.001).
Conclusion: Our cohort study of 26,992 patients with SpA receiving a first biologic drug demonstrated better drug retention with anti-IL17 than with anti-TNF, regardless of SpA phenotype and independent of potential prescribing bias.
To cite this abstract in AMA style:
Pham T, Curmin R, FLACHAIRE B, Tubach F, MICELI C. Drug Survival in Spondyloarthritis: Anti-IL17 Outperforms Anti-TNF in First Biologic Treatment [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/drug-survival-in-spondyloarthritis-anti-il17-outperforms-anti-tnf-in-first-biologic-treatment/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-survival-in-spondyloarthritis-anti-il17-outperforms-anti-tnf-in-first-biologic-treatment/