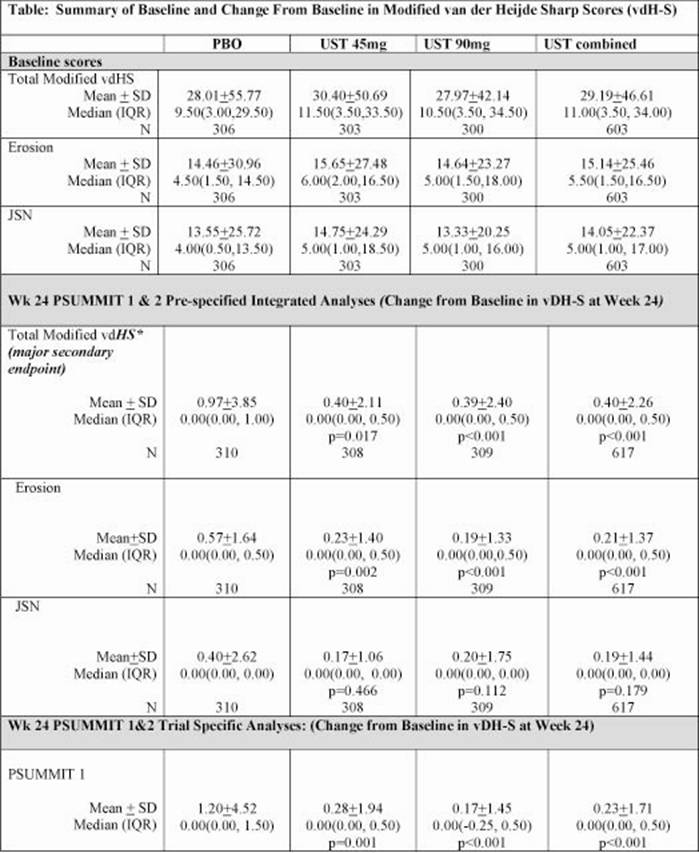
Background/Purpose: We describe the effect of ustekinumab (UST), an IL-12/23 p40 inhibitor, on inhibition of progression of structural damage in patients with active psoriatic arthritis (PsA) at wk24 and wk52 in the PSUMMIT 1 and PSUMMIT 2 trials.
Methods: Adult PsA patients with active disease (≥5 SJC and ≥5 TJC; CRP≥0.3mg/dL [ULN 1.0 mg/dL]) despite DMARD and/or NSAID therapy (PSUMMIT 1, n=615; PSUMMIT 2, n=132) or previously treated with DMARD and/or NSAID, and prior anti-TNFα therapy (PSUMMIT 2, n=180) were randomized to UST45mg, 90mg, or PBO at wks 0, 4, and q12wks, thereafter. At wk16, patients with <5% improvement in TJC & SJC entered blinded early escape [EE] (PBO→UST45mg; UST45mg→90mg; 90mg→90mg). No concomitant DMARDs with the exception of MTX (approximately 50% of patients in each study) were permitted. Radiographs of hands and feet were taken at wks 0, 24, and 52 regardless of EE status, or at the time of study drug discontinuation (unless radiographs were completed within the prior 8wks). Erosions and joint space narrowing (JSN) were evaluated by independent readers blinded to treatment, patient IDs and image time sequence using PsA modified van der Heijde-Sharp (vdH-S) method (total score ranging from 0-528). The major secondary endpoint of change from baseline in total vdH-S scores at wk24 was analyzed based on a pre-specified integrated data analysis using data combined from both studies. Imputation of missing data was done using linear extrapolation or median change of 0.
Results: Baseline disease characteristics including total vdH-S, TJC, SJC and CRP were comparable between PSUMMIT 1 & 2. In the integrated analyses, both UST 45 mg and 90 mg treated patients demonstrated a significant difference in change from baseline in total vdH-S scores at wk24 vs PBO (Table). Moreover, continued inhibition was demonstrated through wk 52; patients randomized to PBO who initiated UST at wk16 or 24 demonstrated slowing of radiographic progression by wk52 (mean change in total vdH-S wk24 to wk52 of 0.08). These observations were reproduced when PSUMMIT 1 was evaluated alone. In PSUMMIT 2, a demonstrable effect of UST on inhibition of structural damage progression could not be discerned; these results may have been impacted by missing radiographic data, especially among PBO-treated patients (23% missing radiographs).
Conclusion: Based upon the pre-specified integrated data analysis, ustekinumab inhibits radiographic progression at wk24.
Disclosure:
I. B. McInnes,
Novartis, Janssen Research & Development, LLC.,
8,
UCB,
9;
C. T. Ritchlin,
Amgen, Janssen, UCB, Abbott (AbbVie), Regeneron,
5,
Amgen, Janssen, UCB,
2;
P. Rahman,
Amgen, Abbott, BMS, Merck, Pfizer, Janssen, Hoffman-La Roche, UCB, Novartis, Sanofi-Aventis,
5,
Amgen, Abbott, BMS, Merck, Pfizer, Janssen, Hoffman-La Roche, UCB, Novartis, Sanofi-Aventis,
9;
L. Puig,
Abbvie, Amgen, Janssen, Lilly, Novartis, Pfizer, VBL,
2,
Celgene, Abbvie, Janssen, Novartis, MSO, Pfizer, Lex Pharma,
5;
A. B. Gottlieb,
Janssen, Amgen, Abbott (Abbvie), Novartis, Celgene, Pfizer, Lilly, Coronado, Levia,
2,
Astellas, Janssen, Celgene Corp., Bristol Myers Squibb Co., Beiersdorf, Inc., Abbott Labs. (Abbvie), TEVA, Actelion, UCB, Novo Nordisk, Novartis, Dermipsor Ltd., Incyte, Pfizer, Canfite, Lilly, Coronado, Vertex, Karyopharm, CSL Behring Biotherapies for Li,
5;
M. Song,
Janssen Research & Development, LLC.,
3;
B. Randazzo,
Janssen Research & Development, LLC.,
3;
S. Li,
Janssen Research & Development, LLC.,
3;
Y. Wang,
Janssen Research & Development, LLC.,
3;
A. M. Mendelsohn,
Janssen Research & Development, LLC.,
3;
A. Kavanaugh,
Janssen Research & Development, LLC.,
9.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ustekinumab-is-effective-in-inhibiting-radiographic-progression-in-patients-with-active-psoriatic-arthritis-integrated-data-analysis-of-two-phase-3-randomized-placebo-controlled-studies/