Session Information
Date: Monday, November 18, 2024
Title: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Postmenopausal osteoporosis (PMOP) significantly contributes to global fracture rates annually, with its silent progression complicating early detection and intervention. Effective management strategies are crucial, including both non-pharmacological and pharmacological approaches. Despite many randomized clinical trials (RCTs) targeting PMOP, numerous trials remain unpublished or discontinued, hindering scientific progress and patient care. This study aims to analyze the prevalence, characteristics, and publication history of PMOP clinical studies and investigate reasons for their non-publication or discontinuation.
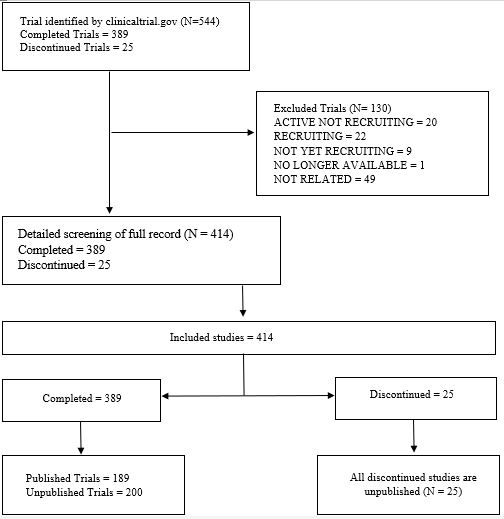
Methods: We thoroughly searched clinicaltrials.gov for PMOP trials up to December 31, 2021. We excluded studies completed within the last 24 months to allow for ongoing peer review. Using NCT identifiers, we identified published trials. Data on gender, age, study type, funding, intervention, enrollment, and location were extracted (Figure 1). Multiple logistic regression analyzed characteristics linked to unpublished or discontinued trials.
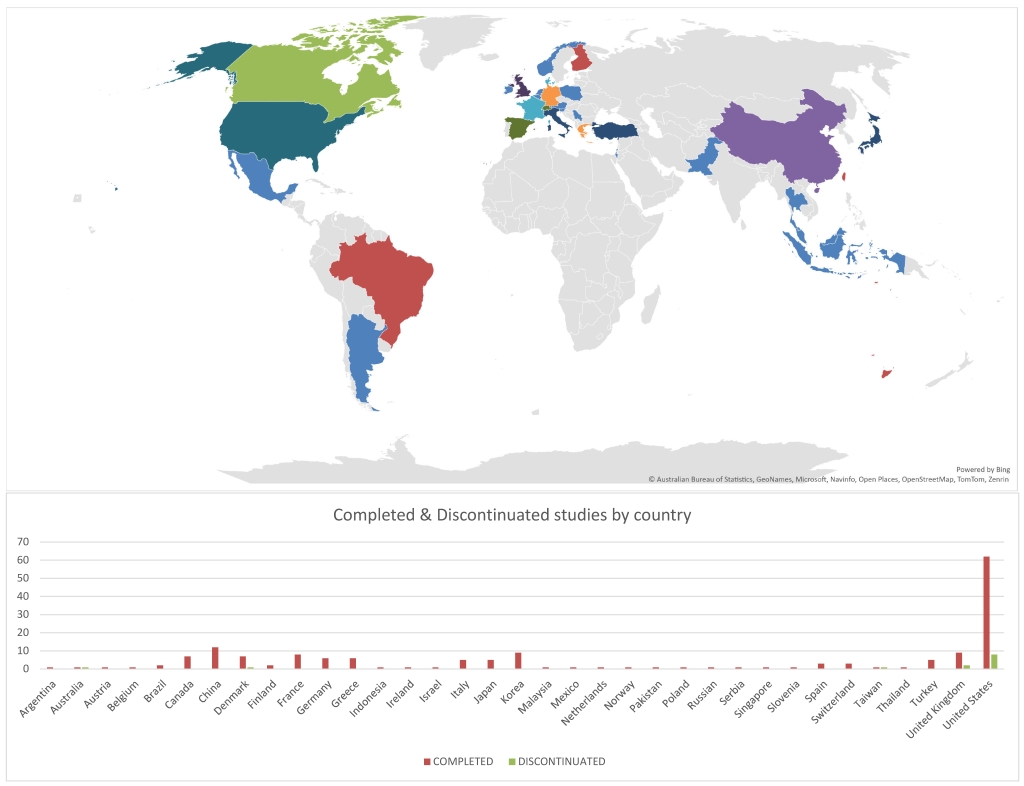
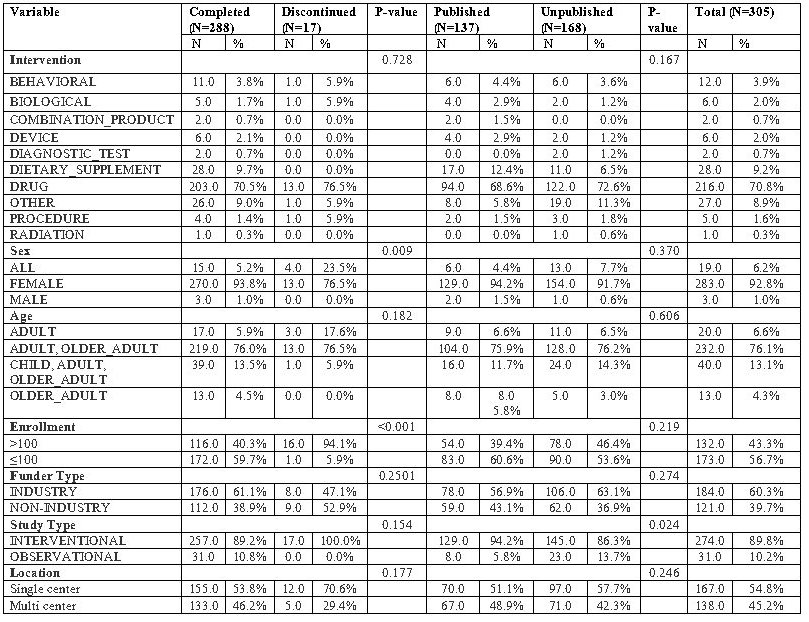
Results: The search identified 414 trials focusing on PMOP. The main study type was interventional studies 362 (87.4%), with drug intervention being the majority 293 (73.6%). More than half of studies recruited over one hundred patients 240 (59.6%), with industrial fund being the primary source 254 (61.4%). 345,435 patients participated in all trials with completion of 389 (93.96%). However, twenty-five studies were discontinued with an exposure of 17,582 patients (Table 1). Moreover, more than half of all trials (54.3%) remained unpublished, exposing 215,833 patients to unnecessary risk. Of the discontinued studies, 16 (64%) were terminated, and the remaining were withdrawn. The logistic regression analysis revealed that studies with an enrollment of less than one hundred participants (OR =0.0589, 95% CI [-4.932, -0.733], p = 0.008) were associated with significantly lower odds of completion. Meanwhile, studies funded by non-industry sources had significantly lower odds of publication (OR = 0.487, 95% CI [-1.346, -0.0937], p = 0.024). Notably, the United States had the highest number of discontinued studies (61.54%) despite only conducting (38.2%) of PMOP total studies (Figure 2).
Conclusion: This is the foremost crosssectional analysis of PMOP clinical studies. The analysis revealed that two-thirds of the patients were enrolled in either discontinued or non-published PMOP clinical studies and that the United States lead the world in the number of discontinued PMOP studies. The high incidence of unpublished and discontinued studies results in exposing participants to potentially ineffective or harmful interventions without generating useful findings. To enhance trial outcomes and improve patient care in PMOP management, future clinical studies must address the barriers of low enrollment and insufficient funding. Addressing these issues is essential for advancing scientific knowledge and optimizing the effectiveness of PMOP interventions.
To cite this abstract in AMA style:
Abdelsalam M, Alabdallat y, Hussein Y, Sajed M, Taha M, morse R, Selima W, Zabady A. Discontinuation and Non-publication of Postmenopausal Osteoporosis Clinical Studies: A Cross-sectional Analysis of 345,435 Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/discontinuation-and-non-publication-of-postmenopausal-osteoporosis-clinical-studies-a-cross-sectional-analysis-of-345435-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-and-non-publication-of-postmenopausal-osteoporosis-clinical-studies-a-cross-sectional-analysis-of-345435-patients/