Session Information
Date: Monday, November 18, 2024
Title: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoporosis (OP) is one of the most common diseases, especially in old age, and as the population ages, the burden of osteoporosis is expected to rise, emphasizing the need for effective treatments and preventive measures. The discontinuation and non-publication of clinical trials not only represents a loss of scientific knowledge but also exposes participants to unnecessary risks without yielding benefits. This study aims to analyze the prevalence, characteristics, and publication history of OP clinical studies and investigate reasons for their non-publication or discontinuation.
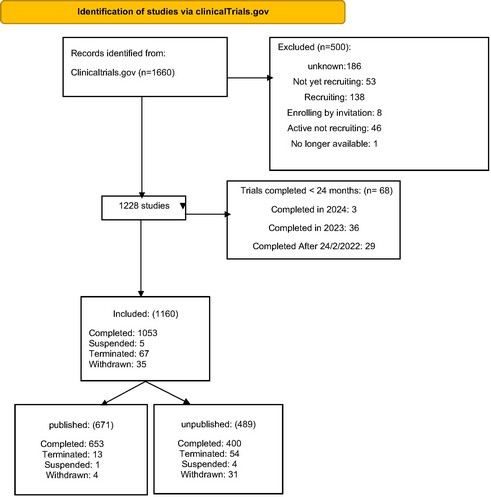
Methods: We thoroughly searched clinicaltrials.gov for osteoporosis trials up to February 2nd, 2022. We excluded studies completed within the last 24 months to allow for ongoing peer review. Using NCT identifiers, we identified published trials. Data on gender, age, study type, funding, intervention, enrollment, and location were extracted (Figure 1). Multiple logistic regression analyzed characteristics linked to unpublished or discontinued trials.
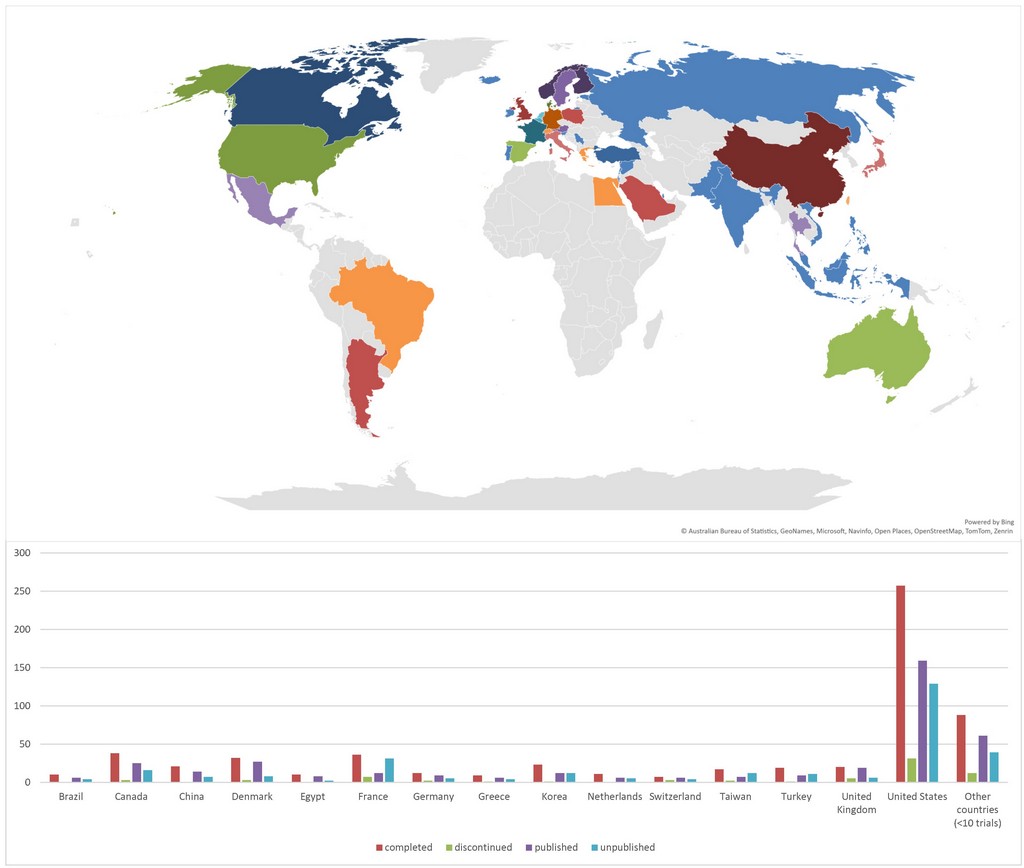
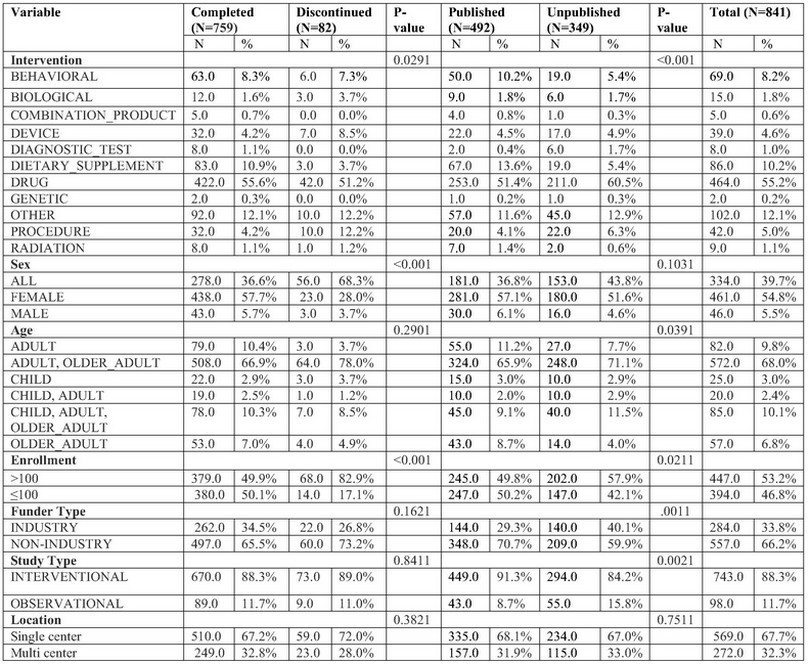
Results: Our analysis included 7,670,624 participants enrolled across 1160 studies, 107 (9.2%) studies were discontinued, with recruitment-related issues and lack of funding financial being the main reasons (P< 0.001) (Table 1). 489 (42.2%) were unpublished, exposing 890,744 patients to unnecessary risks. 632 (54.5%) studies were primarily female. 799 (67.2%) studies recruited “Adult, Older Adults”, 772 (66.6%) had non-industrial sponsor. 583 (50.3%) of the trials had sample size ≥ one hundred patients. The majority 901 (77.7%) were interventional studies, with drug intervention present in 580 (50%) (Table 1). Surprisingly, 18 (16.7%) of discontinued trials were published. The logistic regression analysis revealed that studies included female participants and studies with enrollment size above one hundred patients had significantly lower odds of discontinuation (Odds Ratio (OR) = 0.2952, 95% Confidence Interval (CI) [0.1689 – 0.513]) (P < 0.001) and (OR= 0.1768, 95% CI [0.0932 – 0.336]) (P < 0.001). Studies sponsored by non-industrial entities and studies with enrollment size above one hundred patients have significantly lower incidence of being unpublished (OR = 0.488, 95% CI [0.3287 -0,724]) (P < 0.001) and (OR = 0.594, 95% CI [0.4328 – 0.816]) (P = 0.001).Conversely, observational studies had higher odds of not being published (OR = 1.709, 95% CI [1.0576 – 2.762]) (P = 0.029) (Table 3). The United States, despite only conducting 228 (33.38%) of clinical studies, has the highest discontinuation (45.58%) and nonpublication rate (43.43%) globally (figure 2).
Conclusion: This is the foremost crosssectional analysis of OP clinical studies. The United States has the highest number of discontinued & non-published OP studies globally. Future studies should seek Enhancing recruitment strategies, securing diverse funding sources, and prioritizing the publication of all trial results to optimize clinical trial efficacy and impact. Researchers should refrain from publishing discontinued work without marking it to prevent tenting the clinical data pool with low quality or biased work.
To cite this abstract in AMA style:
Abdelsalam M, al-Najjar A, Samy o, morse R, Rafat r, Sajed M. Discontinuation and Non-Publication of Osteoporosis Studies: An Observational Analysis of 7,670,624 Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/discontinuation-and-non-publication-of-osteoporosis-studies-an-observational-analysis-of-7670624-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-and-non-publication-of-osteoporosis-studies-an-observational-analysis-of-7670624-patients/