Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Certain psoriatic disease treatments, including TNF-α inhibitors (TNFi), increase risk of latent tuberculosis infection (LTBI) activation.1,2 Current treatment guidelines recommend TB screening before initiating systemic therapy.3,4 Here, we reported safety outcomes in LTBI+ patients (pts) with moderate-to-severe psoriasis (PsO) or active PsA who received guselkumab (GUS) for up to 5 years (yrs).
Methods: Safety data were pooled from 11 phase 2/3 studies (7 PsO, 4 PsA). GUS was administered as 100 mg subcutaneous injections – PsO studies: at Week (W)0, W4, then every 8 weeks (q8w); PsA studies: at W0, W4, then q4w/q8w. Pts randomized to placebo (PBO) crossed over to GUS at W16 in PsO studies and at W24 in PsA studies. Pts were screened for TB at baseline. Pts with active TB were excluded. Pts with LTBI were eligible if appropriate LTBI treatment was to be initiated prior to/with the first study drug administration, or if appropriate treatment had been completed within 5 yrs. Safety was reported for PBO-controlled period, yr-by-yr, and through the end of follow-up (PsO: up to 5 yrs; PsA: up to 2 yrs).
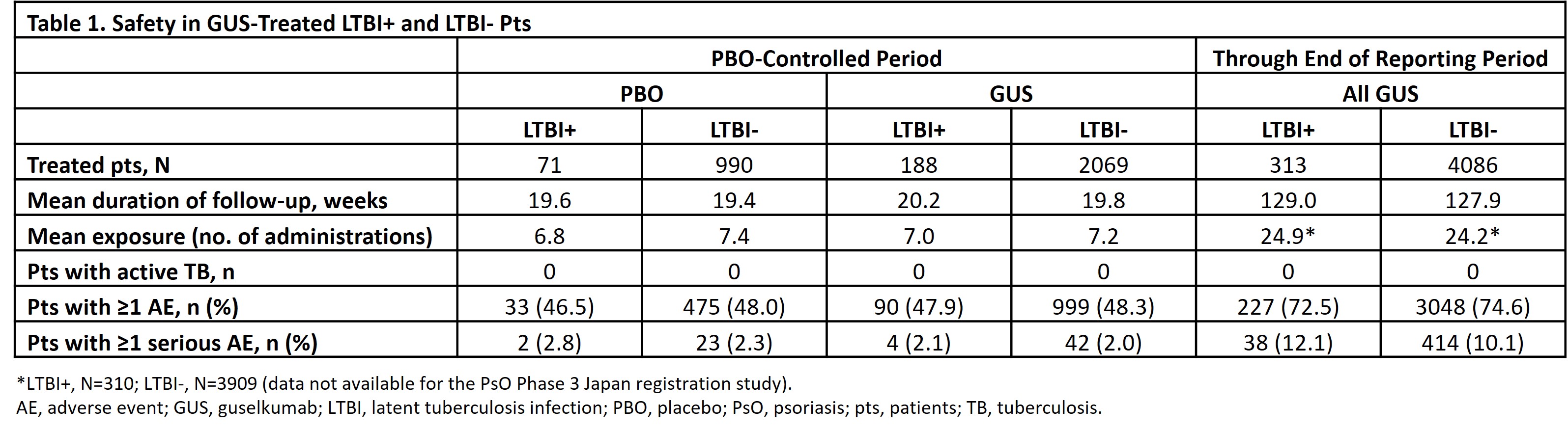
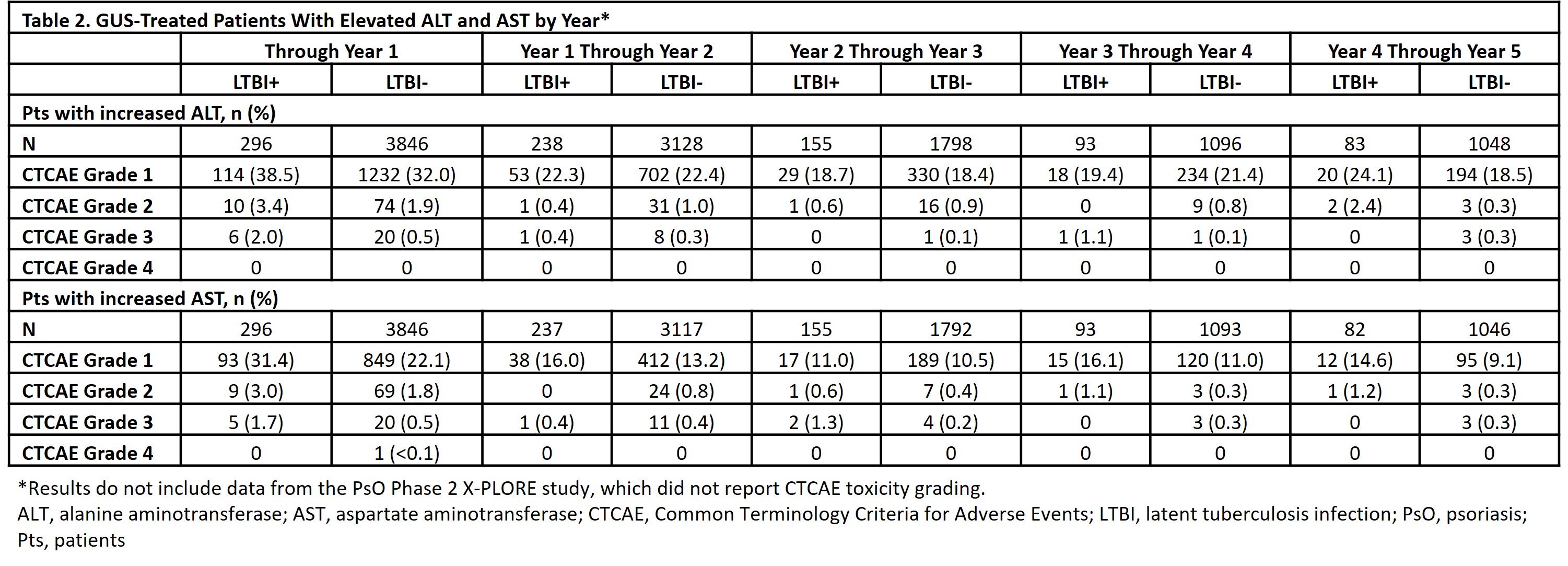
Results: Among all randomized pts, 10.0% (70/697) from Asia-Pacific, 7.3% (51/698) from Western Europe, 7.3% (179/2453) from Eastern Europe, and 5.3% (74/1407) from North America had LTBI. LTBI treatment initiation occurred prior to (88.2% [330/374]), with (6.1% [ 23/374]), or after (5.6% [21/374]) the first dose of study drug (median, -8.0 days; interquartile range [IQR], -20.0 to -2.0). LTBI treatments included isoniazid (82.1%), rifampicin (11.8%), and other medications (17.4%); 89.8% received monotherapy. No new-onset TB/LTBI activation was observed in any GUS-treated pt. During PBO-controlled period, rates of adverse events (AEs) and serious AEs were similar for GUS- and PBO-treated pts in the LTBI+ and LTBI- groups (Table 1). Through the end of follow-up, GUS-treated LTBI+ and LTBI- pts had similar cumulative rates of AEs and serious AEs. Through Yr 1, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations were more common in the LTBI+ vs LTBI- group; ≤2% of LTBI+ pts had CTCAE Grade 3 elevations vs 0.5% of LTBI- pts (no LTBI+ pts had Grade 4 elevations) (Table 2). From Yr 1-5 (after ~98% of LTBI+ pts completed prophylaxis [median (IQR) treatment duration=185 (124-274) days]), proportions of LTBI+ pts with elevated ALT/AST were generally similar to the LTBI- group.
Conclusion: No new-onset TB/LTBI activation was observed in up to 5 yrs of GUS treatment. GUS safety was generally similar in LTBI+ and LTBI- pts. Consistent with the known safety of LTBI medications,5 ALT/AST elevations were more common in LTBI+ vs LTBI- pts through Yr 1; ≤2% of LTBI+ pts had Grade 3 vs 0 had Grade 4 elevations. Rates of ALT/AST elevation were generally similar in LTBI+ and LTBI- pts post-LTBI treatment. The absence of observed TB risk in GUS-treated pts suggests GUS may be a better treatment option than TNFi in high-risk pts, including those in TB-endemic regions.
1Kaushik SB. J Am Acad Dermatol. 2019; 80:43-53.
2Gialouri CG. Mediterr J Rheumatol. 2022; 33:150-61.
3Menter AM. J Am Acad Dermatol. 2019; 80:1029-72.
4Coates LC. Nat Rev Rheumatol. 2022; 18:465-79.
5Tostmann A. J Gastroenterol Hepatol. 2008; 23:192-202.
To cite this abstract in AMA style:
Puig L, Tsai T, Soriano E, Bhutani T, Miller M, Kollmeier A, Li S, You Y, Petrick M, Li H, Patel H, Lavie F, Gooderham M, Londoño A, Lebwohl M, Nash P. Safety in Patients with Latent Tuberculosis Who Received Concomitant Anti-Tuberculosis Medications: Analysis of 11 Studies of Guselkumab in Psoriatic Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-in-patients-with-latent-tuberculosis-who-received-concomitant-anti-tuberculosis-medications-analysis-of-11-studies-of-guselkumab-in-psoriatic-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-in-patients-with-latent-tuberculosis-who-received-concomitant-anti-tuberculosis-medications-analysis-of-11-studies-of-guselkumab-in-psoriatic-disease/