Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Pericarditis is inflammation of the pericardial sac which can be of infectious or non-infectious etiology, commonly associated with rheumatic diseases. Pericarditis is defined as “recurrent” in cases of relapse after a minimum symptom-free interval of 4–6 weeks. 1,2 In the past few years, multiple case reports were published showing that pericarditis cases increased due to long-lasting COVID-19 symptoms and mRNA COVID vaccination. 3-5 First-line treatments include Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), colchicine and low-dose steroids which can all be used as monotherapy or in combination. Patient comorbidities play a significant role in choice of therapy due to safety concerns. Rilonacept is an IL-1 inhibitor and is the first FDA approved treatment (2021) for recurrent pericarditis. To date, there is no published data that provides a standardized approach to utilize rilonacept for recurrent pericarditis. More data is needed to evaluate the long-term efficacy and safety of rilonacept and to provide guidelines for treatment.
This study aims to investigate trends in patient characteristics, treatment patterns including initiation, corticosteroid use and transition process from anakinra, clinical improvement, and safety. Also to provide a standardized approach to tapering NSAIDs, colchicine, and glucocorticoids and a standardized process of transition from anakinra to rilonacept.
Methods: The is a clinical practice pattern retrospective observational study including all patients treated with rilonacept for recurrent pericarditis in Albany Medical Center – Division of Rheumatology. Statistical analysis is mainly descriptive using means, percentages, and standard deviations.
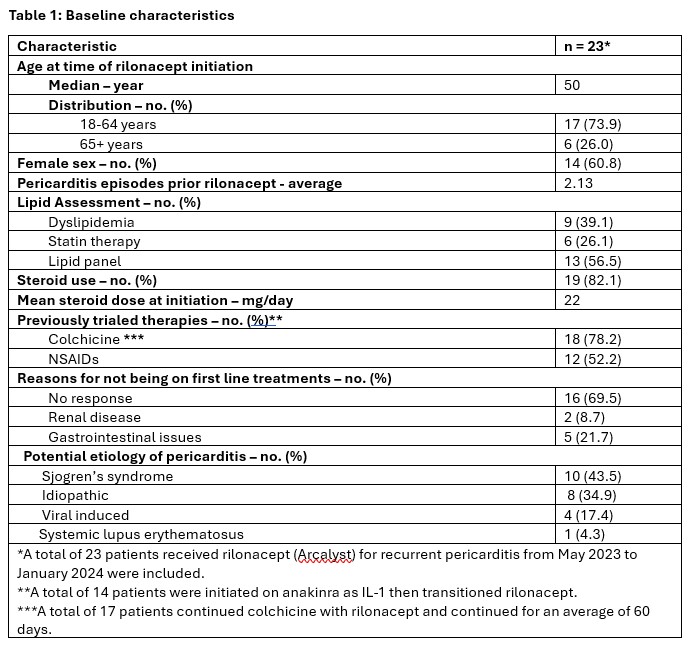
Results: A total of 23 patients who received rilonacept for recurrent pericarditis from May 2023 to January 2024 were included (Table 1). There have been no occurrence of recurrent pericarditis after rilonacept initiation. Corticosteroids were tapered over an average of 6.6 weeks after rilonacept initiation. Out of 23 patients, a total of 19 patients continued rilonacept dosing every 7 days, doses were extended to every 10 days in 3 patients, and every 3 weeks in 1 patient. Twenty-two patients are still receiving rilonacept, with maximum duration reached up to 106 weeks. One patient discontinued the treatment due to continuous chest pain not related to pericarditis. Per patients report, there have been no severe infections developed during therapy with rilonacept. Pharmacy team was involved in obtaining access to rilonacept and patient education for 100% of patients
Conclusion: In a small population, we did not identify major safety concerns and rilonacept seems to be efficacious as steroid sparing therapy for a treatment duration of more than 100 weeks. Pharmacy team role is significant in terms of medication access, patient and providers education, transition of care including steroid tapering plan and follow up. This study will provide practical guidance on accessing the medication, utilizing rilonacept appropriately, and transition of care including steroids taper and follow up.
To cite this abstract in AMA style:
Farrell J, Allen M, Shapiro L, Michaels V, Javed A, Qureshi N, Afridi S, Almahmoud S, Bosques Gomez G, Ngo K. Clinical Practice Patterns, Rilonacept Appropriate Use for Recurrent Pericarditis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clinical-practice-patterns-rilonacept-appropriate-use-for-recurrent-pericarditis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-practice-patterns-rilonacept-appropriate-use-for-recurrent-pericarditis/