Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Individuals with rheumatic disease receiving certain immunosuppressive agents are at risk for severe COVID-19. However, it is unclear if rheumatic disease treatments affect the duration of SARS-CoV-2 viral shedding. Tumor necrosis factor inhibitors (TNFi) and rituximab (RTX) are common rheumatic disease treatments. RTX, in particular, is associated with a higher risk of severe COVID-19 and a possible risk factor for prolonged viral shedding. We sought to evaluate the association of TNFi and RTX use with duration of viral shedding.
Methods: The POSITIVES study is an ongoing prospective cohort recruiting outpatients with a positive SARS-CoV-2 test, including those using a TNFi at infection or rituximab (RTX) in the previous 12 months. Participants self-collect anterior nasal swabs for viral PCR every 2-3 days for up to 6 samples over 2 weeks or until two consecutive negative SARS-CoV-2 PCR tests. We selected all outpatients receiving TNFi and RTX in the cohort and for each group, conducted 2:1 nearest neighbor propensity score matching on age, sex, race, ethnicity, number of prior COVID-19 vaccinations, and use of antivirals. We used survival methods to compare time to first negative viral PCR between those using immunosuppressants and the matched comparators.
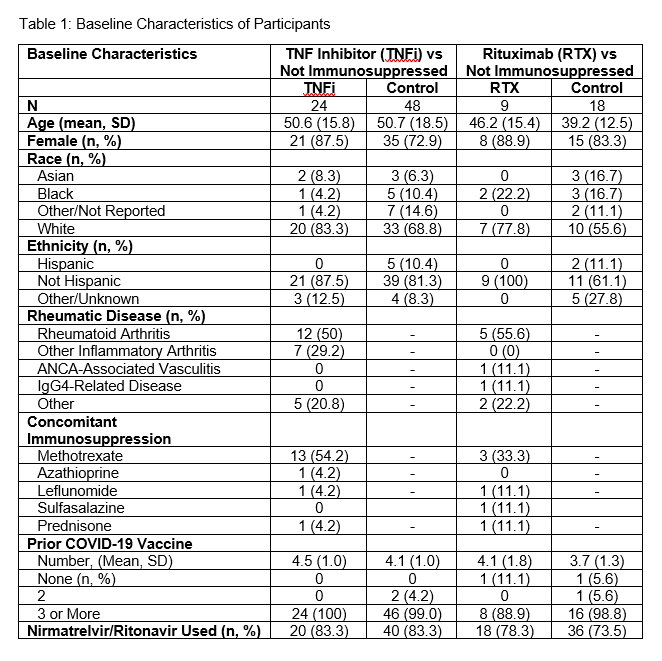
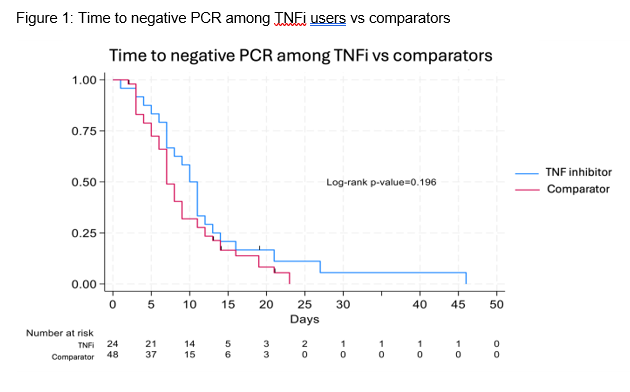
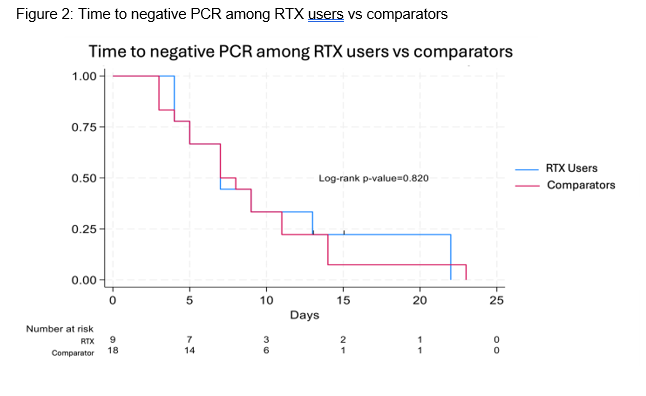
Results: We have enrolled 24 TNFi users and 9 RTX users, matching them to 48 and 18 non-immunosuppressed participants, respectively. Participants were infected between February 2022 and April 2024. Age, sex, number of vaccines, and nirmatrelvir/ritonavir use were similar among TNFi/RTX users and matched comparators (Table 1). Most participants included in this analysis were vaccinated; the mean COVID-19 vaccination number in the TNFi and RTX groups, respectively, was 4.5 (1.0) and 4.1 (1.0), respectively. The median (IQR) time to the first negative PCR was not significantly different when comparing TNFi users vs comparators: 10 (7, 13) vs 7 (5, 11) days, respectively (Figure 1; Log-rank p=0.2). All RTX users had a dose of RTX in the 184 days prior to infection (median days from RTX infusion to infection: 91, range 18-184); the median number of RTX infusions in the 2 years prior to infection was 6 (range 2-13). The median (IQR) time to negative PCR in RTX users vs comparators was also similar: 7 (4.5, 11) vs 7 (5, 11) days, respectively (Figure 2; Log-rank p=0.8).
Conclusion: In this prospective study of outpatients with COVID-19 in the omicron era, TNFi and RTX users had similar durations of viral shedding vs non-immunosuppressed participants. High rates of vaccination, uptake of antivirals, and prior exposure to COVID-19 may improve COVID-19 outcomes in this population, especially RTX users. Assessing the duration of viral culture positivity and differences in viral load are important next steps in this cohort.
To cite this abstract in AMA style:
Wallace Z, Yijia L, Choudhary M, Boucau J, Nathan A, Liew M, Edelstein G, Glover O, Kawano Y, Uddin R, Deo R, Marino C, Getz M, Reynolds Z, Su k, Passell E, Barry M, Gilbert R, Tien D, Sagar S, Vyas T, Hammond S, Vyas J, Gaiha G, Lemieux J, Siedner M, Li J, Barczak A, Sparks J. Duration of SARS-CoV-2 Viral Shedding After Infection Among Patients with Rheumatic Disease Using Tumor Necrosis Factor Inhibitors or Rituximab [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/duration-of-sars-cov-2-viral-shedding-after-infection-among-patients-with-rheumatic-disease-using-tumor-necrosis-factor-inhibitors-or-rituximab/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/duration-of-sars-cov-2-viral-shedding-after-infection-among-patients-with-rheumatic-disease-using-tumor-necrosis-factor-inhibitors-or-rituximab/