Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Cyclobenzaprine is a commonly-used centrally-acting muscle relaxant; it has been approved for short-term treatment of muscle spasms associated with acute musculoskeletal pain despite limited effectiveness.1 The drug is structurally similar to tricyclic antidepressants, raising concerns for tricyclic-like effects on QRS prolongation potentially leading to life-threatening tachycardia and increased mortality.2 Many Veterans suffer from long-term chronic pain; given the trend against prescribing opioids, the use of non-opioid pain medications has increased. Therefore, we aimed to compare mortality rates of new users of cyclobenzaprine or baclofen (an active comparator) in US Veterans with musculoskeletal conditions.
Methods: We conducted a retrospective cohort study using data from the Veterans Health Administration from 2015-2021. We included new users of cyclobenzaprine or baclofen, aged 18-89, with medical codes indicating a diagnosis of musculoskeletal pain in the 90 days prior to prescription fill. We excluded patients with serious illnesses, in long-term care, or who had prior use of the other study drug. The primary outcome was out-of-hospital mortality; the secondary outcome was all-cause mortality. Date of death was obtained from Vitals records. We used Cox proportional hazards regression to estimate the hazard ratios for the study outcomes and inverse probability weighting to control for confounders. To build the propensity score we used 148 variables, which included demographics, comorbidities, and medications.
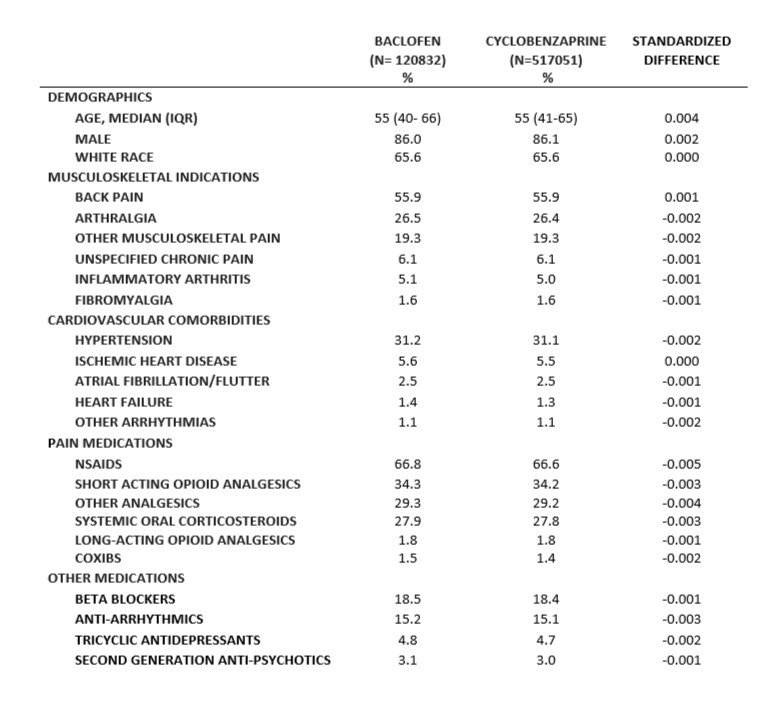
Results: We included 517,051 new users of cyclobenzaprine and 120,832 new users of baclofen. The median age was 55, 86.1% were males, and 65.6% had reported White race (Table). The most common indications were back pain and arthralgia. The most prevalent comorbidities were hypertension, ischemic heart disease, and atrial fibrillation/flutter. There were 1906 out-of-hospital deaths (1302 in the cyclobenzaprine and 604 in the baclofen group), and 2040 overall deaths (1393 in the cyclobenzaprine and 647 in the baclofen group). For out-of-the hospital mortality, the incidence rate in cyclobenzaprine users was 10.26 per 1000 person-years compared to 13.18 in baclofen users, with an unadjusted HR of 0.81 (95% CI: 0.74-0.89). After adjustment for confounders, the HR was 0.96 (95% CI: 0.86-1.06). The rate of all-cause mortality in cyclobenzaprine users was 10.96 per 1000 person-years compared to 14.10 in baclofen users, with a HR of 0.81 (95% CI: 0.74-0.89). After adjustment for confounders, HR was 0.95 (95% CI: 0.86-1.05).
Conclusion: Among US Veterans with chronic musculoskeletal conditions, there was no statistically significant differences in out-of-hospital mortality or all-cause mortality between new users of cyclobenzaprine and baclofen.
To cite this abstract in AMA style:
Intriago M, Nepal P, Daniel L, Dickson A, Wilson O, Stein C, Hung A, Chung C. Mortality in US Veterans with Musculoskeletal Conditions Using Cyclobenzaprine or Baclofen [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/mortality-in-us-veterans-with-musculoskeletal-conditions-using-cyclobenzaprine-or-baclofen/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mortality-in-us-veterans-with-musculoskeletal-conditions-using-cyclobenzaprine-or-baclofen/