Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Recently, the European Medicines Agency (EMA) published a health alert that extended to all JAK inhibitors (JAKi) due to a possible class effect, recommending avoiding the use of these drugs in patients aged 65 years or older, cardiovascular risk, smokers or ex-smokers and those with a high risk of cancer, allowing their use only when there is no other treatment alternative. This study aims to determine the proportion of patients who have developed any cardiovascular, ischemic, neoplastic, or thrombotic adverse event in a cohort of patients receiving or who have received JAKi therapy. In addition, we studied the impact of the alert in the clinical practice of our Rheumatology department.
Methods: Retrospective, observational, descriptive, and longitudinal study. Patients from the Rheumatology department of the Hospital Universitario Infanta Sofía who received or have received treatment with JAKi between January 1, 2017, and September 30, 2023, were included. Demographic variables such as age and sex, and clinical variables such as medical history, rheumatologic diagnosis, duration of JAKi use, adverse events, and reasons for JAKi change were collected. Additionally, we evaluated all patients treated with JAKi after the date of publication of the mentioned EMA alert, all patients who met the alert criteria were informed and were offered another therapeutic option, if possible.
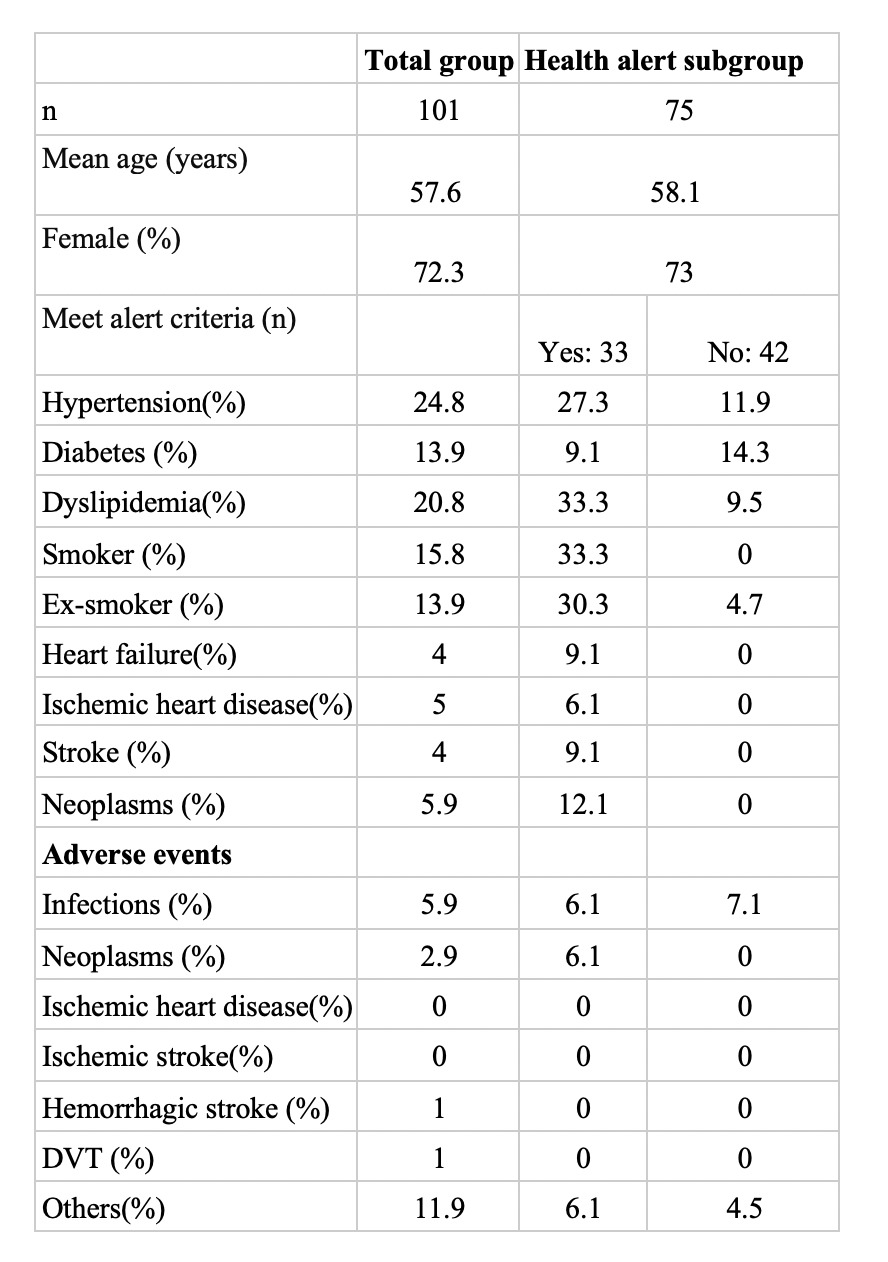
Results: A total of 101 patients were analyzed. The majority were women (72.3%), with a mean age of 57.6 years. Most frequent comorbidities were hypertension, diabetes and dyslipidemia, 15.8% were active smokers, and 13.9% were ex-smokers. Regarding the diagnoses 60.4% had rheumatoid arthritis, 18.8% had psoriatic arthritis, 5.9% had axial spondyloarthritis. Of the total of patients 29.7% were on treatment with Baricitinib, 6.9% with Filgotinib, 10.8% with Tofacitinib, and 52.5% with Upadacitinib. The median duration of all the drugs used was 26 months. The most frequent adverse events observed were severe infections in 5.9%, neoplasms in 2.9%, deep vein thrombosis in 1%, hemorrhagic stroke in 1% (anticoagulated patient). No patient developed an ischemic stroke or an ischemic heart disease during JAKi use.
In 2022 when the health alert was published by the EMA, 75 patients were on treatment with JAKi, of whom 33 met the alert criteria. Among these patients who met the alert criteria, 12.1% changed the treatment because of the alert, and 12.1% due to treatment failure, while 9.1% were maintained because there were no other therapeutic options, 57.6% because of being in clinical remission or low disease activity, and 9.1% at patient request. All patients in whom JAKi were maintained, had agreed to continue with this treatment.
Conclusion: Despite the inferred risk associated with the use of JAKi, no new cardiovascular events or ischemic strokes were recorded in our group of patients in current clinical practice. This finding is consistent with the latest data published in the RABBIT study. In addition, the majority of patients treated with JAKi maintained treatment in spite of the EMA alert. However, due to the relatively short follow-up period in our study, it would be desirable to increase the number of patients in order to draw stronger conclusions.
To cite this abstract in AMA style:
Castañeda Estévez E, Vergara Dangond C, Steiner M, Paredes Romero M, Esteban Vázquez A, Cobo Ibañez T, Trives Folguera L, Romero Bogado M, De La Cámara Fernandez I, Richi Alberti P, Acosta Alfaro A, De La Osa Subtil I, Muñoz Fernández S. Impact in Clinical Practice of the European Medicines Agency Health Alert About the Restriction of the Use of JAK Inhibitors [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-in-clinical-practice-of-the-european-medicines-agency-health-alert-about-the-restriction-of-the-use-of-jak-inhibitors/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-in-clinical-practice-of-the-european-medicines-agency-health-alert-about-the-restriction-of-the-use-of-jak-inhibitors/