Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: Pain in Rheumatic Disease Including Fibromyalgia
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: A consistent line of investigation proposes fibromyalgia as a stress-evoked sympathetically maintained neuropathic pain syndrome. This theory posits dorsal root ganglia (DRG) at the epicenter of fibromyalgia pain [1]. DRG house the soma of the somatosensory neurons filtering to the brain painful stimuli arising from most parts of the body including internal organs. Each individual neuronal nucleus tightly interacts with its enveloping immune-competent satellite glial cells (SGCs). Different mechanical and inflammatory mediators activate SGCs inducing cytokine release and stronger SGCs-nociceptive neuron coupling leading to chronic pain [2].
Little is known about the acute effect of human serum on cultured murine DRG nociceptive cell physiology. IgG from fibromyalgia patients induce hyperalgesia in mice by binding to SGCs [3].
Objectives: To define if human serum can acutely activate murine DRG pro-nociceptive cells. To find out if the serum of women suffering from fibromyalgia induces more pronounced acute stimulation of DRG neurons and/or their SGCs when compared to serum from healthy women.
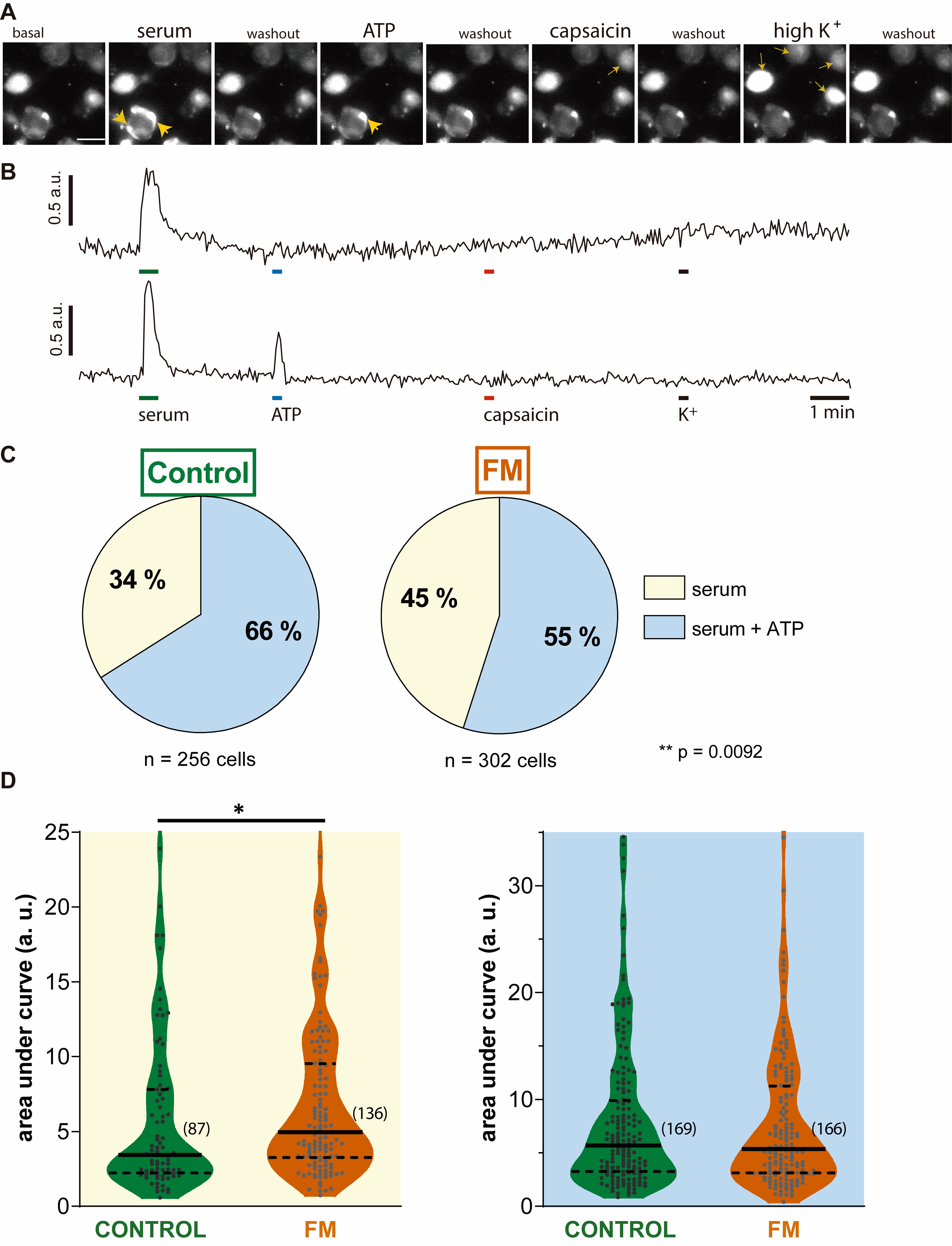
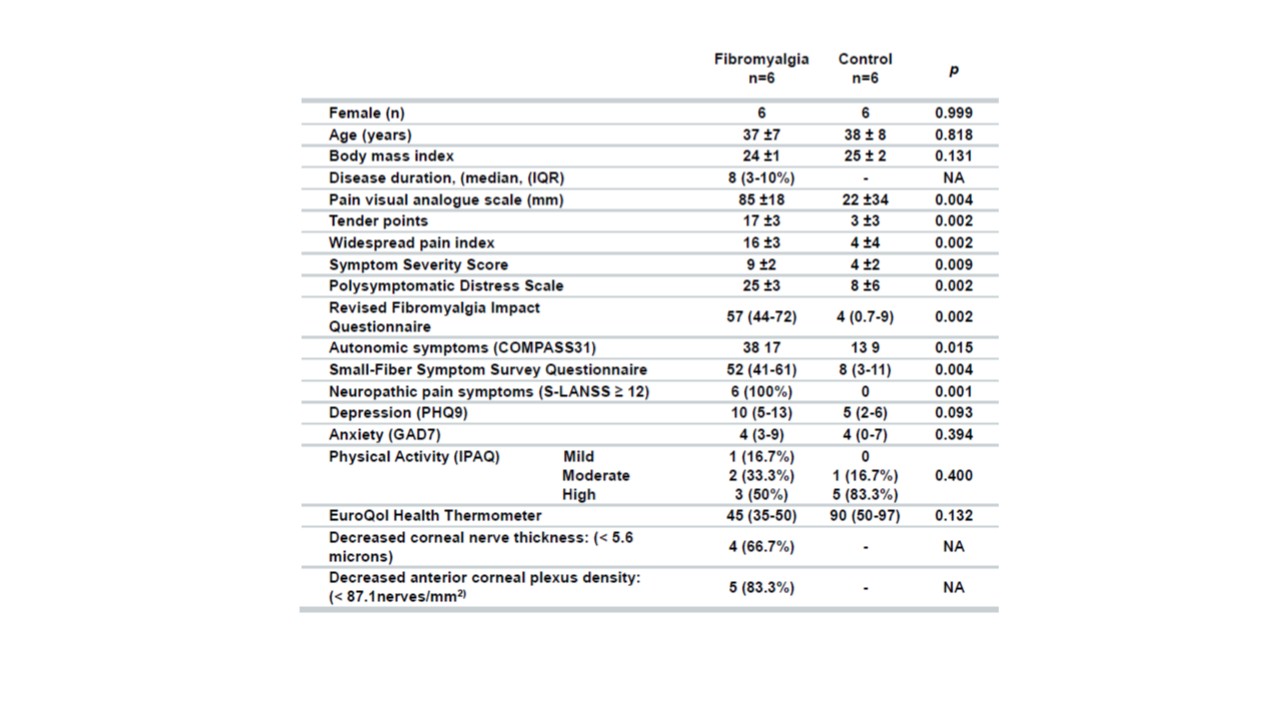
Methods: Serum from 6 women suffering from fibromyalgia and 6 matched healthy controls were tested on Wistar rat DRG neurons and their SGCs. The serum and the following activating substances; capsaicin 1 µM, ATP 10 µM and K+ 60 mM, were sequentially perfused onto primary DRG cell cultures. Fluo-4 was used as intracellular calcium concentration reporter. The magnitude of the response of DRG nociceptive cells to the different activating components was defined as the fluorescence change (∆F/F0) area under the curve. The number of SGCs activated by human serum but not by ATP (ATP unresponsive) was quantified in order to explore if the cell activation could be independent of P2X7 signaling. Researchers were blinded to the serum provenance.
Results: 1477 DRG neurons were studied, of these 625 were activated by human serum. The intensity of neuronal activation in response to patients’ serum was not different from controls (Mann-Whitney U p = 0.1646). The percentage of neurons activated by patients serum (43%) was not different from controls (41%) p = 0.492.
558 DRG SGCs were activated by human serum. A higher proportion of patients’ serum (45%) induced ATP unresponsive SGCs activation compared to controls’ serum (34%) (Fisher’s exact test, p = 0.0092) (Figure). Patients serum induced greater calcium influx into SGCs unresponsive to ATP with an area under the curve = 4.95 (3.27 – 9.5) median (quartile 25 – 75) vs. 3.4 (2.23 – 7.8) (Mann-Whitney U, p = 0.0122).
Conclusion: Human serum is able to acutely activate murine DRG pro-nociceptive cells. When compared to serum collected from healthy women, serum derived from fibromyalgia sufferers induces more intense and widespread stimulation on ATP unresponsive DRG SGCs, suggesting the presence of potentially identifiable pro-nociceptive substance(s) in the circulation. SGCs hyperstimulation appears to be independent of P2X7 signaling. DRG SGCs may play an important role in the pathogenesis of fibromyalgia.
Ref: [1] Martínez-Lavin. Clin Rheumatol 2021;40:783 [2] Hanani & Spray. Nat Rev Neurosci. 2020;21:485 [3] Goebel et al J Clin Invest. 2021;131.e144201
To cite this abstract in AMA style:
Mercado F, Segura-Chama P, Almanza A, Martinez-Martinez L, Martinez-Lavin M. Acute Activation of the Rat Dorsal Root Ganglion Nociceptive Cells by the Serum of Patients Suffering from Fibromyalgia [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/acute-activation-of-the-rat-dorsal-root-ganglion-nociceptive-cells-by-the-serum-of-patients-suffering-from-fibromyalgia/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/acute-activation-of-the-rat-dorsal-root-ganglion-nociceptive-cells-by-the-serum-of-patients-suffering-from-fibromyalgia/