Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Biological agents such as the TNF-α inhibitors have had a positive impact on the treatment of many chronic autoimmune diseases (AIDs), such as Rheumatoid Arthritis (RA)1. However, access to these biologic therapies varies globally, and drug availability is limited in many countries due to drug costs. Biosimilars are reproductions of their originator counterparts, are usually less expensive and therefore provide a potential opportunity to improve patient access2.
The NOR-SWITCH study, is one the few studies that examined the impact of switching from an originator product to biosimilar in stable patients, it showed that switching from originator to biosimilar infliximab was not inferior to continued treatment with the originator and supported switching for nonmedical reasons, that is to reduce costs3.
This study evaluated the consequences of switching originator biological therapies to its biosimilars in a Mexican cohort of AIDs patients.
Methods: We conducted a longitudinal cohort, including 73 patients from the rheumatology outpatient clinic of the Hospital Regional 1° de Octubre with the following diagnosis: RA, Systemic Lupus Erythematosus (SLE), Ankylosing Spondylitis (AS) and Psoriatic Arthritis (PsA).
Patients who were switched from an originator biological to its biosimilar were followed up every 3 months; biological therapies included were: Adalimumab (ADA), Rituximab (RTX) and Etanercept (ETN), with their biosimilars Amgevita, Maball and Infinitam, respectively. Disease activity was assessed by a rheumatologist and, in every visit, patients were questioned regarding the presence of adverse events (AEs) related to the application of the biosimilar.
Results: Of the 73 patients, 68 were women (93.2%), the most frequent diagnosis was RA in 52 patients (71.2%), followed by 5 (6.8%) with PsA, and 4 patients with AS (5.5%). Forty-seven patients (64.4%) were treated with ADA, 18 (24.7%) with RTX and 8 patients (11%) with ETN.
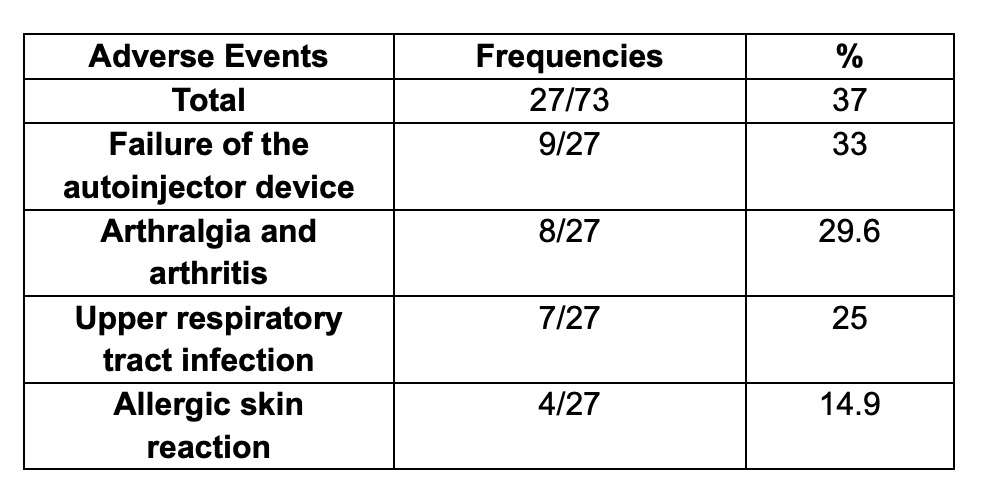
Twenty-seven patients (37%) presented an AEs, 9 patients (33%) had problems with the application of the biosimilar due to failure of the autoinjector device, followed by 8 patients (29.6%) that presented arthralgia and arthritis (considered as an AE but reflecting lack of efficacy), 7 patients (25%) presented upper respiratory tract infection and 4 patients presented an allergic skin reaction (14.8%).
Thirteen patients (19.2%) presented AEs or lack of efficacy using a biosimilar which merited a change in therapy.
Conclusion: Switching of originator biological therapies to its biosimilar in our cohort of patients might not have an impact on efficacy or the incidence of infections; however, one of the most common AEs was associated with the mechanics of the autoinjector device. Although this may seem a minor issue, it led to patients missing a dose, thus having an impact on treatment compliance and thus the activity of the disease.
To cite this abstract in AMA style:
Carrillo S, Xibille D, Castañeda-Martínez M, Reyes A, garcia-Arellano B, Vega Chávez E, Montiel Castañeda C, Carmona Lara G, De Hoyos G. Consequences of Switching Originator Biological Therapies to Its Biosimilars in Patients with Immune-mediated Diseases in a Mexican Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/consequences-of-switching-originator-biological-therapies-to-its-biosimilars-in-patients-with-immune-mediated-diseases-in-a-mexican-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consequences-of-switching-originator-biological-therapies-to-its-biosimilars-in-patients-with-immune-mediated-diseases-in-a-mexican-cohort/