Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Vaccines for SARS-CoV-2 have greatly reduced COVID-19 morbidity and mortality through the induction of neutralizing antibody responses. However, T cell responses are also induced by infection and vaccination and may be particularly important in protecting against severe infection in individuals who lack B cell responses. We have previously shown that SARS-CoV-2 vaccination induces enhanced T cell responses in pharmacologically B-cell depleted patients, potentially contributing to a reduced risk of severe disease. However, the protective capacity of T cell responses to prevent severe outcomes after breakthrough infection in immunosuppressed individuals remains unclear. Here, we longitudinally evaluated T cell responses before and after breakthrough infection in immunosuppressed individuals and their association with disease severity.
Methods: A subgroup of participants from the ACV01 trial were included; ACV01 assessed the response to booster vaccine in people using immunosuppressing therapies for rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, multiple sclerosis, or pemphigus. All patients had an inadequate antibody response to prior SARS-CoV-2 vaccine. Here, we assessed participants with a breakthrough infection between 9/1/2021-3/1/2023 and PBMC samples available pre- and post-infection. Ex vivo T cell reactivity was measured by IFN-γ ELISpot and proliferation through a six-day cell trace dilution-based assay with overlapping SARS-CoV-2 Spike pools containing peptides from the Wuhan Hu-1 (WT), JN.1, EG.5, XBB.1.5 and Omicron (BA.2 or BA.4/5) sequences.
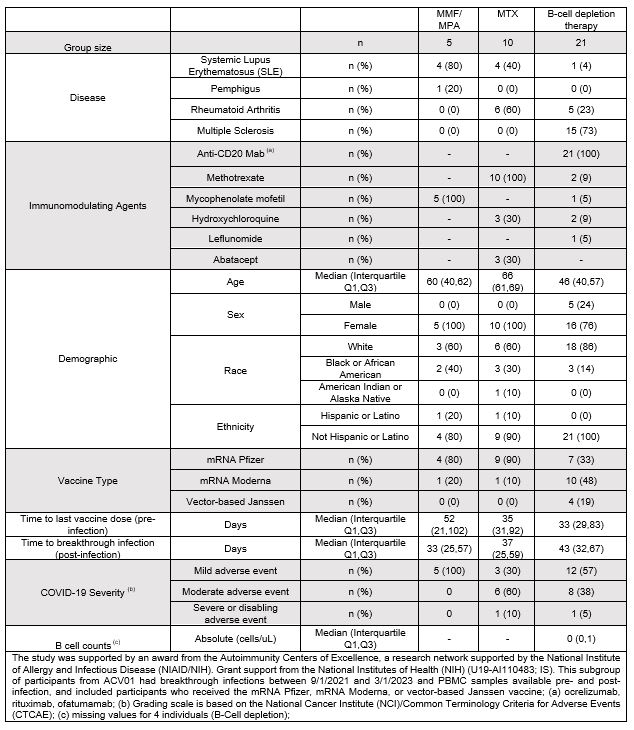
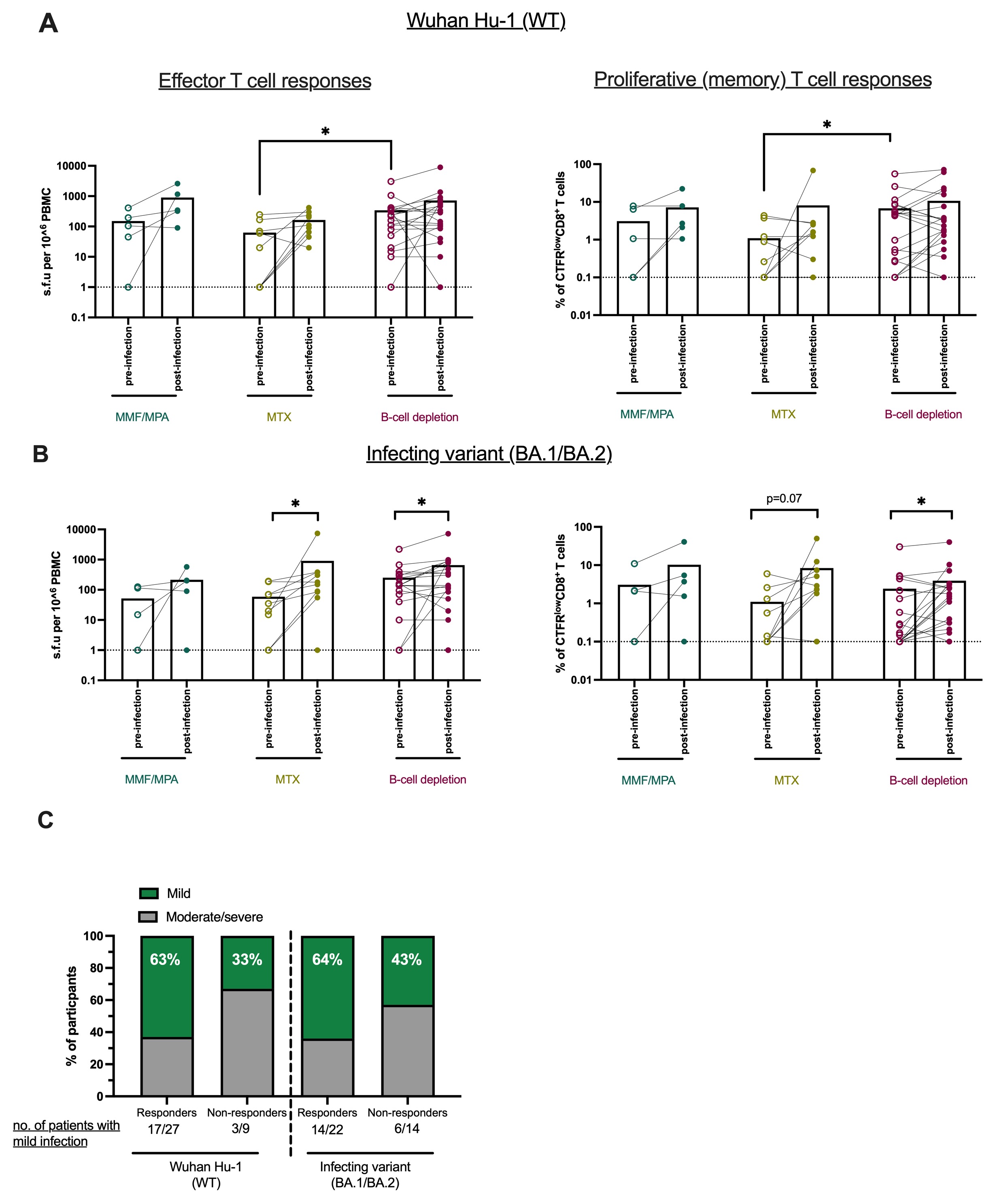
Results: Among the 36 ACV01 trial participants with breakthrough infection and PBMCs, 5 were treated with mycophenolate mofetil (MMF)/mycophenolic acid (MPA), 10 with methotrexate (MTX), and 21 with B-cell depletion therapies (Table 1). Breakthrough infections primarily occurred during the Omicron wave. Pre-infection, B cell-deficient individuals had elevated effector and proliferative CD8+ T cells compared to participants using MMF/MPA or MTX (Figure 1). Although most participants had proliferative CD8+ T cell responses to SARS-CoV-2 WT strain pre-infection, 50% of MTX (5/10) and 45% of B-cell depletion individuals (10/22) had no detectable responses to BA.1/BA.2 Omicron variant. However, breakthrough infection induced CD8+ T cell responses to the BA.1/BA.2 in 40% (2/5) of MTX donors and 50% of B cell-deficient donors (5/10) who lacked responses pre-infection. Breakthrough infection also broadened CD8+ T cell responses to cross-recognize the new dominant XBB.1.5, EG.5 and JN.1 lineages. Evaluation of clinical outcomes of breakthrough infection showed that most immunosuppressed individuals who had a proliferative CD8+ T cell response (27/36) to ancestral SARS-CoV-2 or to BA.1/BA.2 variant (17/27) had mild disease and this was particularly notable amongst B cell-deficient individuals.
Conclusion: We demonstrate the potential role of T cells in limiting COVID-19 disease severity after breakthrough infection in immunosuppressed individuals, highlighting the importance of considering T cell immunity in future SARS-CoV-2 vaccine development.
(A-B) Analysis of effector T cell responses (shown as IFNγ ELISpotspot-forming units (s.f.u) per 106 peripheral blood mononuclear cells “PBMCs”) and proliferative CD8+ T cell responses (% of CTFRlowCD8+ T cells) pre- and post-infection to Wuhan Hu_1 (WT) (A) and BA.1/BA.2 Omicron variant (B).
(C) Comparison of clinical outcomes after BTI in individuals with or without detectable proliferative CD8+ T cell responses (threshold=1% of CTFRlowCD8+ T cells). Differences between groups were evaluated using the nonparametric Mann-Whitney U test. Wilcoxon signed-rank test was used for paired analysis (pre- and post-infection). Calculated P values are presented as follows: *P < 0.05.
To cite this abstract in AMA style:
Alrubayyi A, Shulkin A, James J, Mackay M, Khanna D, Bar-Or A, Macwana S, Goldmuntz E, McNamara J, McCarthy S, Sherman M, Barry W, Pinckney A, Walker S, Tedeschi S, Sparks J, Wallace Z, Gaiha G. Longitudinal Assessment of CD8+ T Cell Responses to SARS-CoV-2 Pre- and Post-breakthrough Infection and Its Association with COVID-19 Severity in Immunosuppressed Individuals [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/longitudinal-assessment-of-cd8-t-cell-responses-to-sars-cov-2-pre-and-post-breakthrough-infection-and-its-association-with-covid-19-severity-in-immunosuppressed-individuals/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/longitudinal-assessment-of-cd8-t-cell-responses-to-sars-cov-2-pre-and-post-breakthrough-infection-and-its-association-with-covid-19-severity-in-immunosuppressed-individuals/