Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome is a monogenic disease of adults due to acquired somatic mutations of the UBA1 gene. Patients present with a broad range of inflammatory and hematological manifestations, and the prognosis is severe. Due to its recent description, the therapeutic management is poorly codified and only based on small retrospective studies. The aim of this study was to evaluate the efficacy and safety of targeted therapies in a cohort of patients with VEXAS syndrome.
Methods: Multicenter retrospective study conducted within the French national VEXAS study registry between November 2020 and August 2023 including patients with genetically proven VEXAS syndrome who had received at least one targeted therapy. CR was defined by clinical remission, CRP < 10 mg/L and corticosteroid therapy less than 10 mg/day; partial response (PR) by clinical remission, CRP and corticosteroid therapy decreased by 50%; and no response by persistence of clinical activity and/or biological inflammatory syndrome and/or inability to decrease corticosteroid therapy.
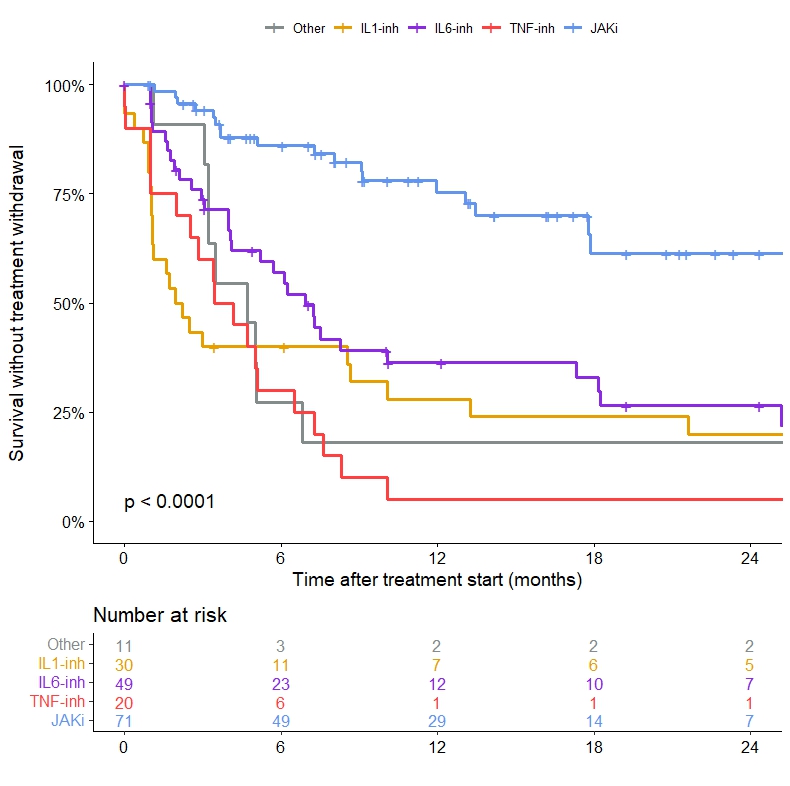
Results: One hundred and ten patients (male 99%, median age 71 (68-79) years) who had received 194 targeted therapies were included. Treatments received were JAK inhibitor (JAKi) in 40% (n=78, ruxolitinib in 87%) of cases, anti-interleukin (IL)-6 antibody in 26% (n=51, tocilizumab in 92%), anti-IL-1 in 17% (n=33, anakinra in 91%), anti-TNFa in 10% (n=20), and another targeted therapy in 6% (n=12). Fifty-three patients (48%) received more than one targeted therapy. The main clinical features of VEXAS syndrome at the initiation of targeted therapy were constitutional symptoms (82%), skin involvement (76%) and inflammatory arthralgias (60%). Myelodysplastic syndrome was present in 28% of cases, without indication for specific hematological treatment. Median CRP at treatment initiation was 39 (19-57) mg/L and median prednisone dose received was 20 (10-35) mg/day. At 3 months, overall response (CR and PR) was 24% (CR 19% and PR 5%) with JAKi, 32% (CR 20% and PR 12%) with anti-IL-6, 9%% with anti-IL-1, and 0% with anti-TNF or another targeted therapy. At 6 months, the overall response was 30% (CR 26% and PR 4%) with JAKi and 26% (CR 20% and PR 6%) with anti-IL-6. Corticosteroid withdrawal was similar between JAKi and anti-IL-6. Compared to JAKi, other therapies had significantly higher risk of treatment withdrawal during follow-up (P< 0.001, Figure 1). Treatment was stopped in 28% (n=22) of cases under JAKi with a median delay of 7.2 (3.4-12.4) months and in 69% of cases under anti-IL-6 with a median delay of 5.1 (2-9.1) months. The causes of discontinuation were primary failure, secondary failure, serious adverse event or death respectively in 43%, 14%, 19% and 19% with JAKi, 46%, 11%, 31% and 9% with anti-IL-6, 50%, 4%, 35% and 8% with anti-IL-1, and 74%, 5%, 16% and 5% with anti-TNFa.
Conclusion: This large multicenter cohort is the first to compare the efficacy and safety of targeted therapies in VEXAS syndrome. This study confirms the benefit of JAKi and tocilizumab with similar response rates, whereas the other targeted therapies appear to have lower efficacy. These results need to be confirmed in prospective therapeutic trials.
To cite this abstract in AMA style:
HADJADJ J, NGUYEN Y, MOULOUDJI D, BOURGUIBA R, HEIBLIG M, HASSINA A, LACOMBE V, ARDOIS S, CAMPOCHIARO C, MARIA A, COMONT T, Lazaro E, LIFERMANN F, LE GUENNO G, LOBBES H, Outh R, Campagne J, COUSTAL C, GARNIER A, Jamilloux Y, MEYER A, Abisror N, KOSMIDER O, Jachiet V, FAIN O, Terrier B, Mekinian A, Georgin-Lavialle S. Efficacy and Safety of Targeted Therapies in VEXAS Syndrome: Retrospective Study from the French VEXAS Group [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-targeted-therapies-in-vexas-syndrome-retrospective-study-from-the-french-vexas-group/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-targeted-therapies-in-vexas-syndrome-retrospective-study-from-the-french-vexas-group/