Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: ABBV-599 is a novel combination of elsubrutinib (ELS; a selective BTK inhibitor) and upadacitinib (UPA; a JAK inhibitor) that targets non-overlapping signaling pathways associated with systemic lupus erythematosus (SLE). The objective of this analysis is to report results from SLEek, a phase 2, randomized, placebo (PBO)-controlled, parallel-group, multicenter study evaluating efficacy and safety of ABBV-599 and UPA monotherapy in adults with moderately to severely active SLE (NCT03978520).
Methods: Patients (pts) were randomized 1:1:1:1:1 to once daily (QD) ABBV-599 high dose (HD; ELS 60 mg + UPA 30 mg), ABBV-599 low dose (LD; ELS 60 mg + UPA 15 mg), ELS 60 mg, UPA 30 mg, or PBO. The primary endpoint was the proportion of patients at W24 achieving SLE Responder Index-4 (SRI-4) and steroid dose ≤ 10 mg QD; additional efficacy and safety endpoints through W48 are also reported. The pre-specified 2-sided alpha level was 0.1.
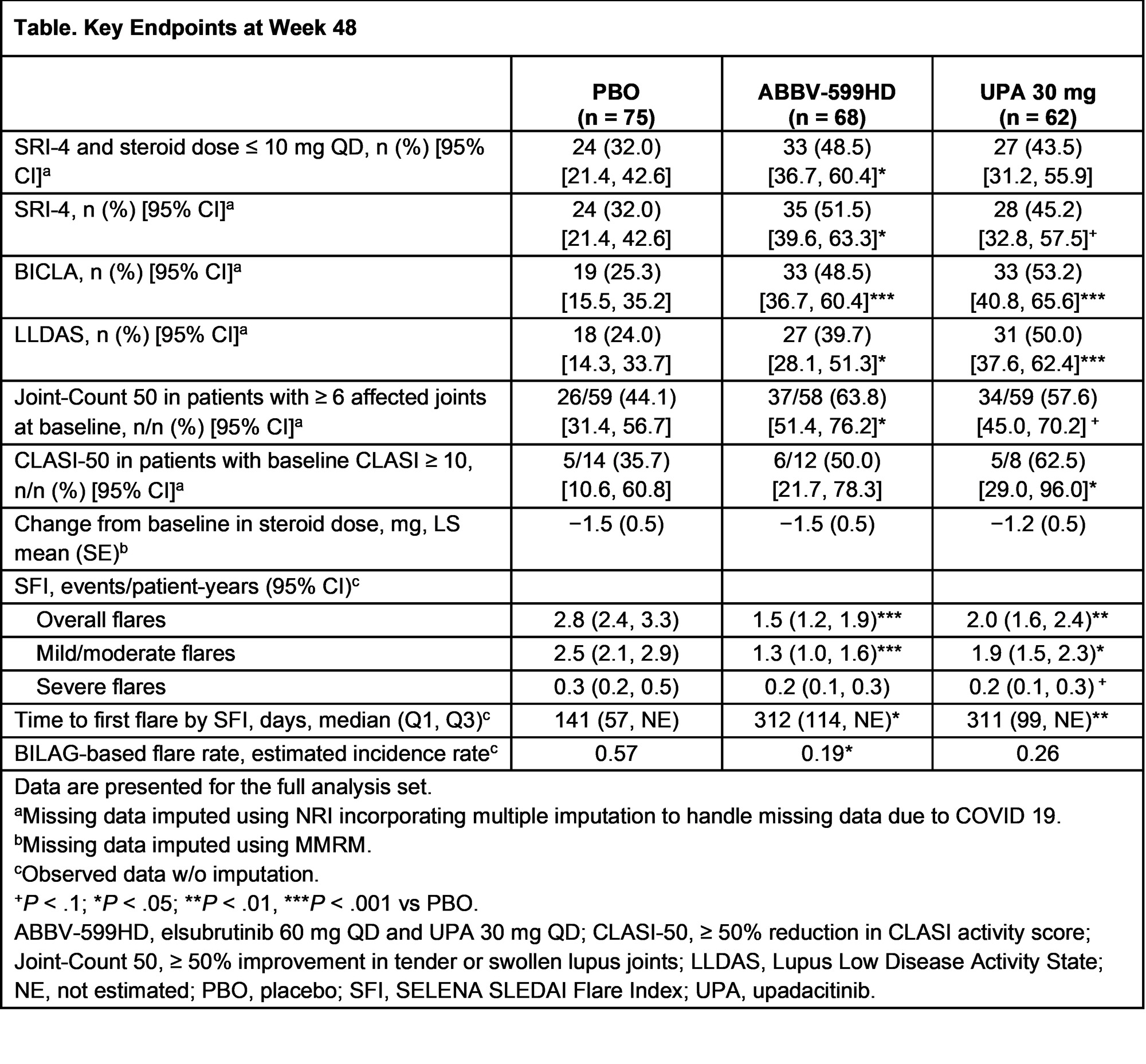
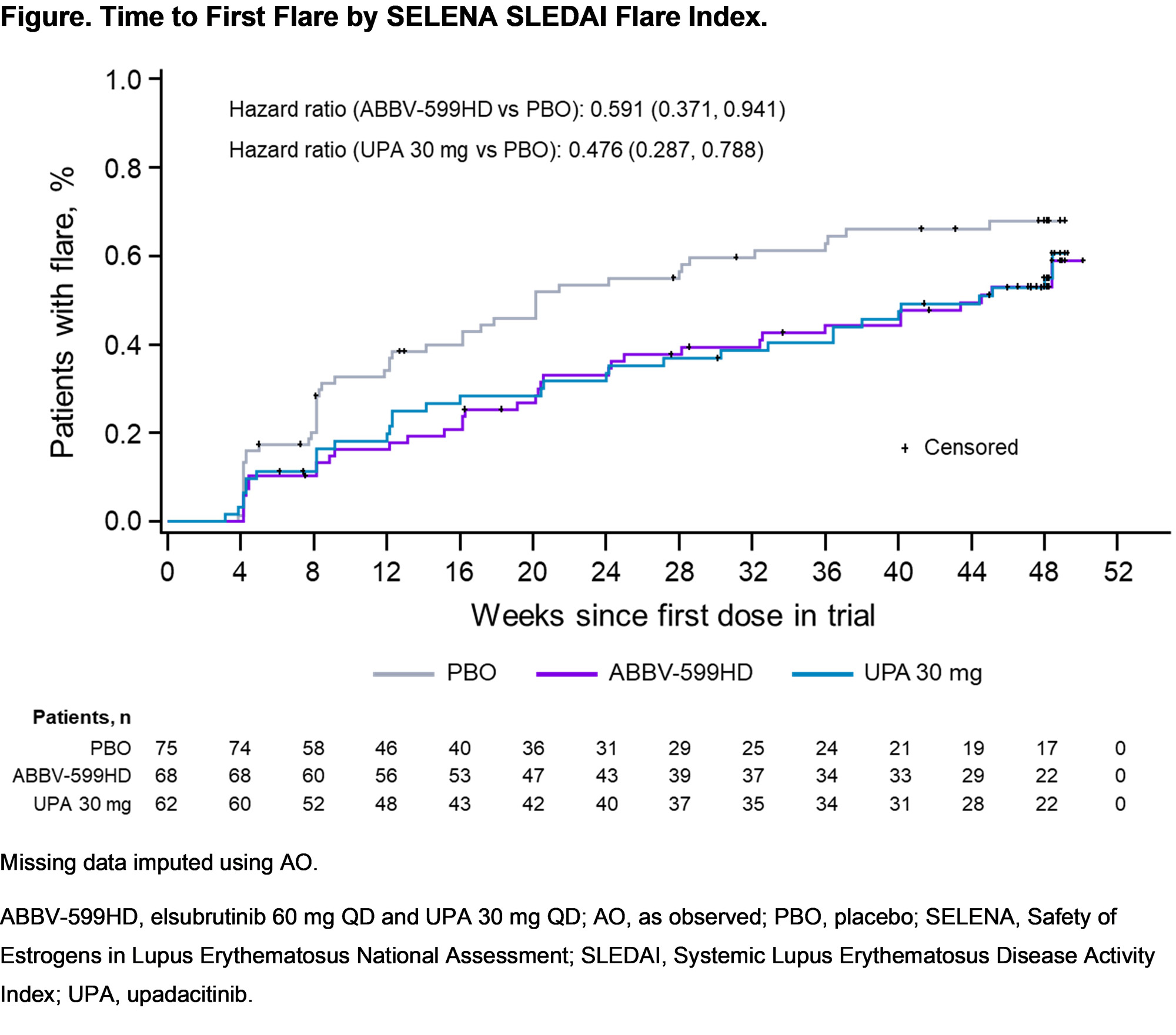
Results: 341 patients were enrolled. After a planned interim analysis when 50% of pts reached W24, the ABBV-599LD and ELS 60 mg arms were discontinued for lack of efficacy (no safety concerns). Of 205 continuing pts (ABBV-599HD n = 68, UPA 30 mg n = 62, PBO n = 75), baseline characteristics were well balanced. The primary endpoint (proportion achieving SRI-4 and steroid dose ≤ 10 mg QD at W24 vs PBO) was met by ABBV-599HD and UPA 30 mg. Key secondary endpoints were also achieved at W48 in both groups (Table). Overall flares and time to first flare were substantially reduced in the ABBV-599HD and UPA 30 mg groups through W48 (Figure). Anti-double stranded DNA antibodies were significantly decreased with both treatments. TEAEs considered related to study drug were 42.6% ABBV-599HD, 32.3% UPA 30 mg, and 33.3% PBO. There were no malignancies or VTE. There were 3 non-fatal CV events (1 MI on PBO and 2 ruptured cerebral aneurysms [1 each on ABBV-599HD and UPA 30 mg]); all were assessed as unrelated to study drug by investigators. No new safety signals were observed beyond previously known data for UPA or ELS.
Conclusion: ABBV-599HD (ELS 60 mg + UPA 30 mg) and UPA 30 mg demonstrated significant improvements in SLE disease activity and flares with acceptable safety through 48 weeks.
To cite this abstract in AMA style:
Merrill J, Tanaka Y, D'Cruz D, Vila-Rivera K, Siri D, Zeng X, D'Silva K, Cheng L, Sornasse T, Doan T, Kruzikas D, Friedman A. Efficacy and Safety of ABBV-599 High Dose (Elsubrutinib 60 mg and Upadacitinib 30 mg) and Upadacitinib Monotherapy for the Treatment of Systemic Lupus Erythematosus: A Phase 2, Double-blind, Placebo-controlled Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-abbv-599-high-dose-elsubrutinib-60-mg-and-upadacitinib-30-mg-and-upadacitinib-monotherapy-for-the-treatment-of-systemic-lupus-erythematosus-a-phase-2-double-blind-placebo-c/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-abbv-599-high-dose-elsubrutinib-60-mg-and-upadacitinib-30-mg-and-upadacitinib-monotherapy-for-the-treatment-of-systemic-lupus-erythematosus-a-phase-2-double-blind-placebo-c/