Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Up to 50% of SLE patients experience neuropsychiatric involvement (neuropsychiatric lupus, or NPLSE) in the form of cognitive deficits, memory loss, depression, and anxiety. The pathogenic mechanisms of NPSLE have yet to be firmly established. Since current non-specific treatment regimens in NPSLE often fail to improve symptoms, identifying novel and more targeted interventions is necessary to improve patient care. Cerebrospinal fluid and serum levels of the inflammatory cytokine interleukin-6 (IL-6) are increased in NPSLE patients and lupus mice. Excessive IL-6, possibly entering the brain from the serum, could promote neuroinflammation by activating glial cells, including astrocytes and microglia. We aimed to assess this putative mechanism, hypothesizing that knocking out IL-6 in lupus mice would ameliorate NPSLE.
Methods: Female MRL/lpr mice exhibit severe lupus-like disease and neuropsychiatric deficits. To investigate the association of IL-6 with NPSLE, we compared serum levels of IL-6 with behavioral testing scores in 18-week-old MRL/lpr mice. Object placement (OP) and object recognition (OR) tests measured novelty preference to assess cognition and memory (increased scores indicate improved performance). Social preference (SP), Porsolt swim, tail suspension, and elevated plus maze (EPM) measured murine correlates of anxiety and depression. We repeated this battery in two separate cohorts of 14- to 18-week-old IL-6 knockout (KO; n = 7 + 8) and age-matched wildtype (WT; n = 7 + 8) MRL/lpr mice. Systemic disease was assessed by serum levels of anti-dsDNA antibodies. We measured gene expression of gfap, an astrocytic activation marker, and aif1, a microglial marker, in right-hemispheric cortical tissue by real-time qPCR. Results were compared between KO and WT using two-tail Mann-Whitney u- or Student’s t-tests when appropriate, with a p-value threshold of 0.05.
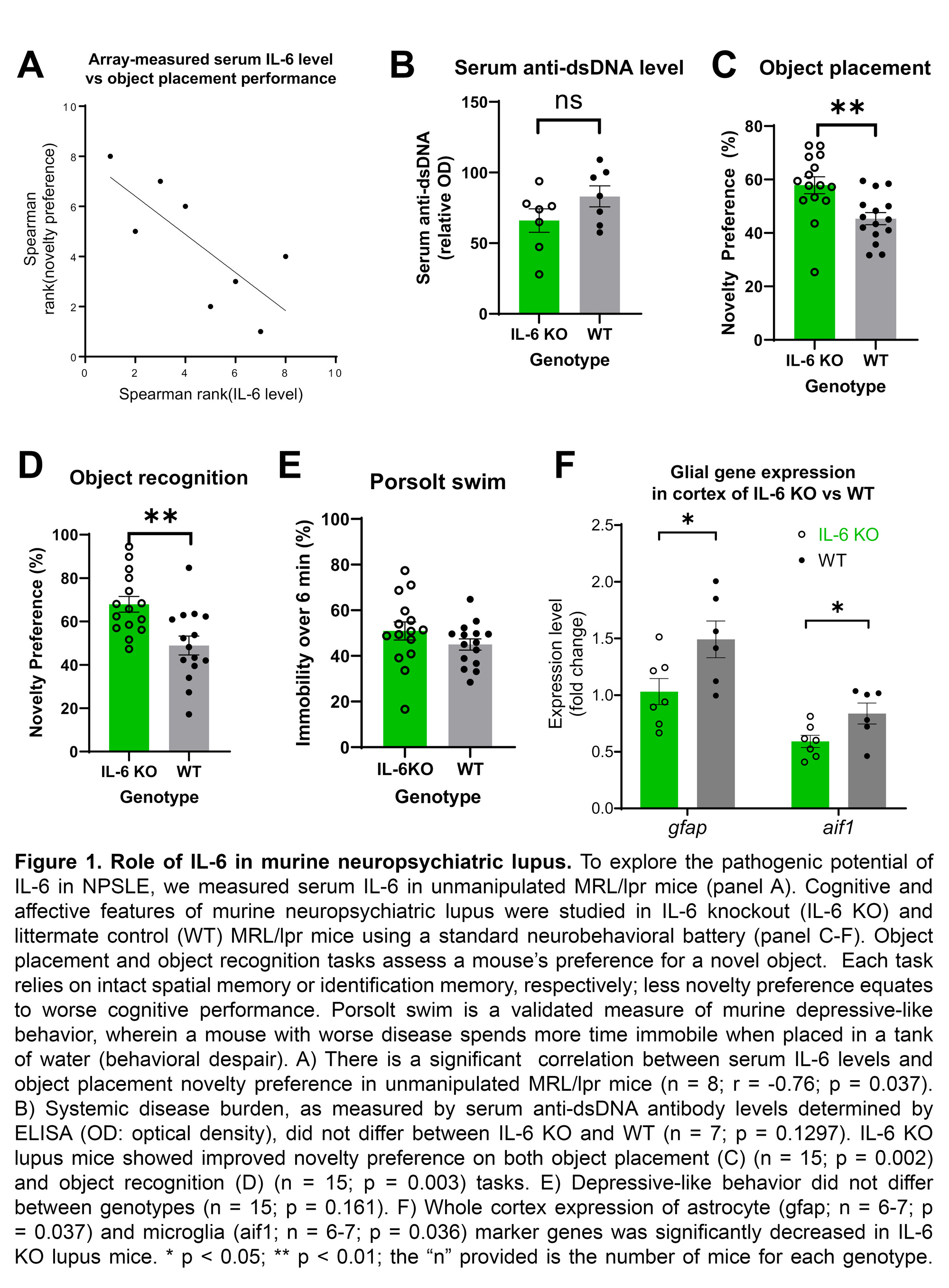
Results: Elevated serum IL-6 correlated with worse spatial memory as measured by the OP task (n = 8; r = -0.76, p = 0.037; Fig 1A), but not with the depression or anxiety tasks. Serum anti-dsDNA antibody titers (Fig 1B) did not significantly differ between IL-6 KO mice and WT controls (p = 0.130). MRL/lpr IL-6 KO mice showed significantly increased novelty preference on OP (KO: 57.87 +/- 3.15; WT: 45.37 +/- 2.29, p = 0.002; Fig 1C) and OR (KO: 67.91 +/- 3.62; WT: 48.93 +/- 4.34; p = 0.003; Fig 1D). No difference was found on SP (p = 0.838), Porsolt swim (p = 0.161; Fig 1E), tail suspension (p = 0.325), or EPM (p = 0.890). Expression of both glial genes, gfap (KO: 1.03 +/- 0.15; WT: 1.49 +/- 0.16; p = 0.037) and aif1 (KO: 0.59 +/- 0.05; WT: 0.84 +/- 0.09; p = 0.036), were decreased in whole cortex samples from IL-6 KO mice compared to WT (Fig 1F).
Conclusion: In conclusion, we found that serum IL-6 levels correlate with worse cognition and memory in lupus mice. Moreover, knocking out IL-6 ameliorates those deficits without altering affective features or systemic disease. Whether pharmacologic inhibition of IL-6 would similarly attenuate murine NPSLE remains to be determined. IL-6 KO mice also demonstrated decreased cortical expression of inflammatory glial genes. Therefore, IL-6 may play a specific, glia-mediated role in the cognitive and memory deficits of NPSLE.
To cite this abstract in AMA style:
Reynolds J, Huang M, Meineck M, Möckel T, Schwarting A, Putterman C. Interleukin-6 Activates Glial Cells and Induces Cognitive Dysfunction in Murine Neuropsychiatric Lupus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/interleukin-6-activates-glial-cells-and-induces-cognitive-dysfunction-in-murine-neuropsychiatric-lupus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interleukin-6-activates-glial-cells-and-induces-cognitive-dysfunction-in-murine-neuropsychiatric-lupus/