Session Information
Date: Tuesday, November 14, 2023
Title: (2387–2424) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Existing EULAR/ACR guidelines on polymyalgia rheumatica (PMR) are focused on the management by rheumatologists. However, there is no consensus regarding early referral and evaluation in secondary care for patients with suspected PMR. It is well known that important differential diagnosis such as giant cell arteritis may be missed in general practice. In addition, a recently conducted worldwide survey suggested a wide heterogeneity in the referral of patients with suspected PMR from primary to secondary care [1]. The aim of this project was to develop evidence-based guidelines for the early referral of patients with suspected PMR.
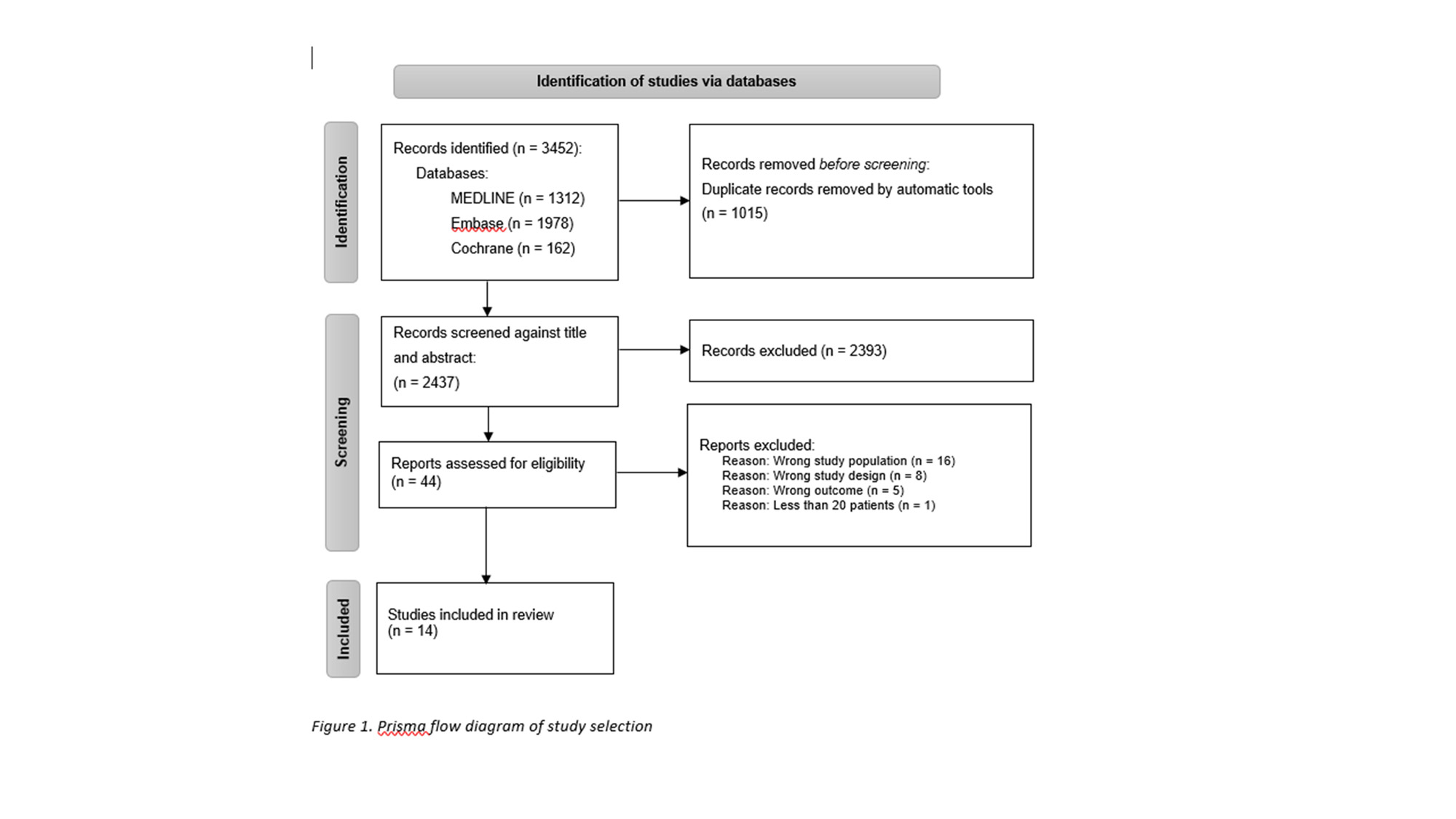
Methods: A task force formed by members from the international giant cell arteritis (GCA)/PMR study group consisting of 38 participants (29 rheumatologists, 4 general practitioners, 4 patients with PMR, and a health care professional) drafted the guideline. Task force activities were led in accordance with the EULAR standard operating procedures. After 3 virtual meetings during 2022, 70 clinical questions were initially identified. These were later reduced to 10 and finally 6 questions following the Population, Intervention, Comparator, Intervention (PICO) format. The protocol for the systematic literature review was published at the PROSPERO database, search were performed on February 14th 2023, and data extraction and evaluation were performed by two investigators. Full text papers with more than 20 participants with suspected PMR evaluating the 6 PICOs were included. The results of the SLR were discussed during 3 online meetings, formulating the draft of the guideline.
Results: The PICO questions are shown in Table 1. The SLR yielded 14 papers, concerning PICO 1, 3, 4, and 6 (Figure 1). For PICO 2 and 5 no studies were identified. Guideline draft included 3 overarching principles and 6 recommendations: mandatory evaluations of patients with suspected PMR in primary care before referral, which patients with suspected PMR to refer for evaluation in secondary care, when to start glucocorticoids in patients referred to secondary care for evaluation of suspected PMR, and when to use rapid refer strategies in patients suspected of PMR, which patients seen in secondary care with suspected PMR should be evaluated for GCA, and which patients could be managed in primary care after the diagnosis.
Conclusion: This is the first international consensus of the management of referral of patients with suspected PMR. The recommendations will ensure a more uniform management in the future, with a decreased risk of misdiagnosis. Moreover, the work will also define the future research agenda in the field.
References [1] Donskov AO, Mackie SL, Hauge EM, et al. An international survey of current management practices for polymyalgia rheumatica by general practitioners and rheumatologists. Rheumatology (Oxford) 2023.
To cite this abstract in AMA style:
Keller K, Mukhtyar C, Nielsen A, Hemmig A, Mackie S, Sattui S, Hauge E, Dua A, Helliwell T, Neil L, Blockmans D, Devauchelle V, Hayes e, Venneboer A, Monti S, Ponte C, De Miguel E, Matza M, Warrington K, Byram K, Yaseen K, Peoples C, Putman M, Lally L, Finikiotis M, Appenzeller S, Carmori U, TORO GUTIERREZ C, Backhouse E, Guerrero M, Pimentel-Quiroz V, Keen H, Owen C, Daikeler T, De Thurah A, Schmidt W, Brouwer E, Dejaco C. Recommendations for Early Referral of Patients with Suspected Polymyalgia Rheumatica: An Initiative from the International Giant Cell Arteritis and Polymyalgia Rheumatica Study Group [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/recommendations-for-early-referral-of-patients-with-suspected-polymyalgia-rheumatica-an-initiative-from-the-international-giant-cell-arteritis-and-polymyalgia-rheumatica-study-group/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recommendations-for-early-referral-of-patients-with-suspected-polymyalgia-rheumatica-an-initiative-from-the-international-giant-cell-arteritis-and-polymyalgia-rheumatica-study-group/